A tetrastyryl cationic fluorescent probe and its preparation method and application
A technology of tetraphenylethylene and fluorescent probes, applied in the field of fluorescent probes, can solve the problems of limited development and inapplicability, and achieve the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
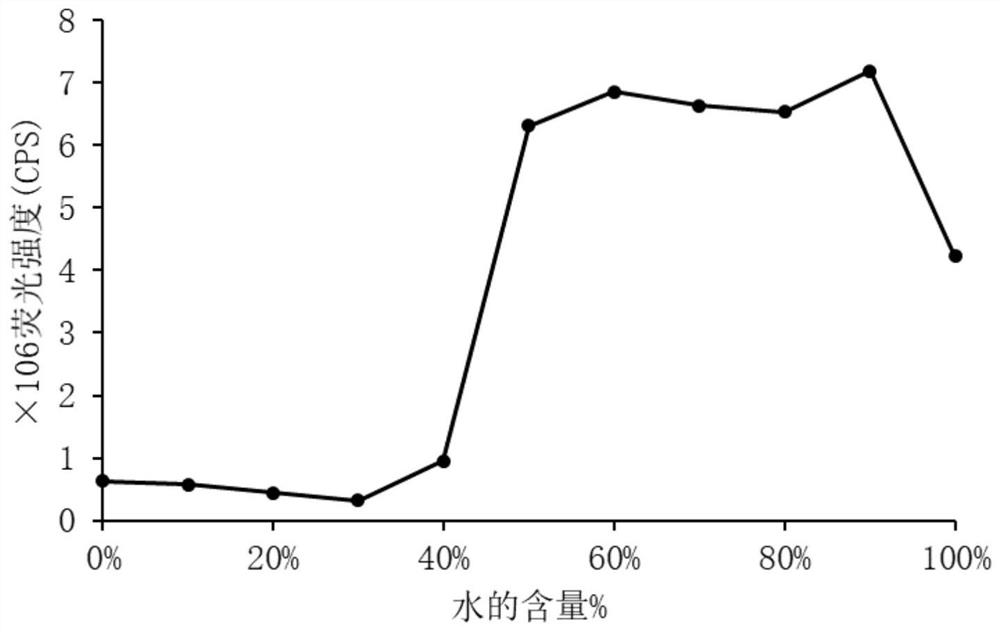
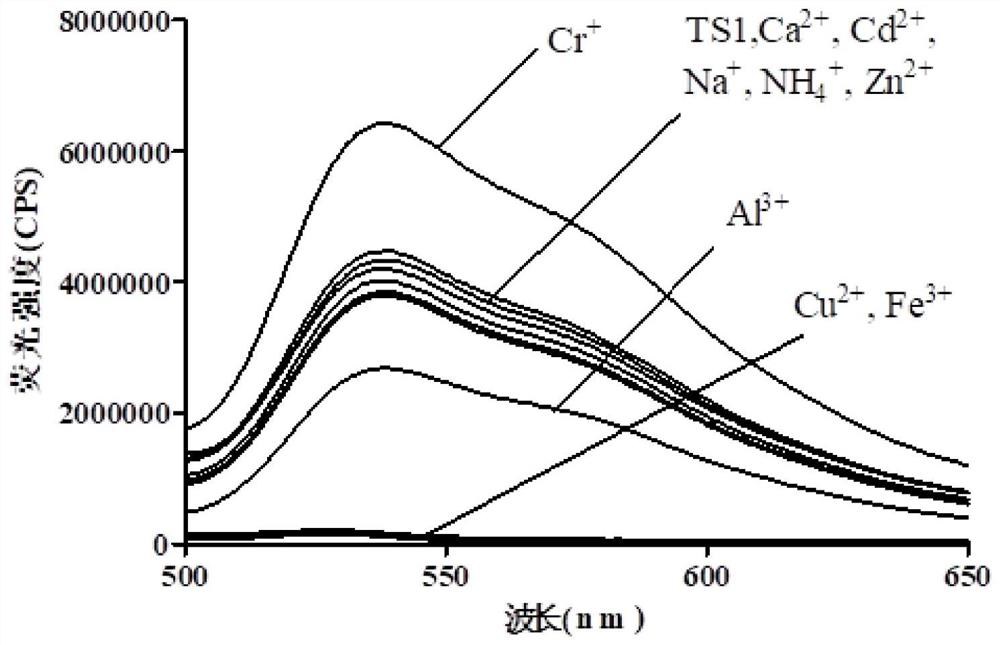
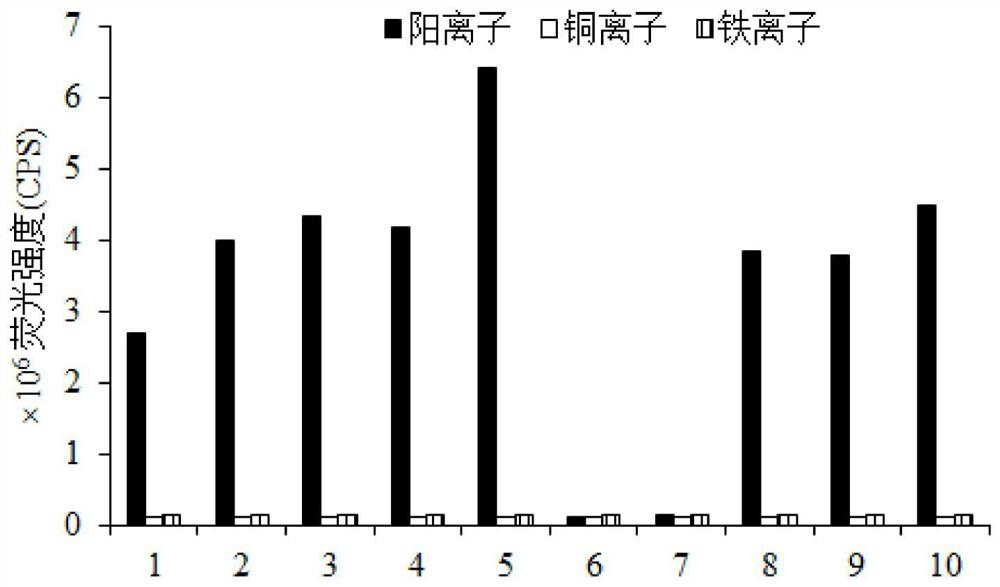
Image
Examples
Embodiment 1
[0044] The following is the structural formula of compound TS1:
[0045]
[0046] The following is the synthetic route of TS1:
[0047]
[0048] (1) Synthesis of compound 1
[0049] Step 1: Add 12mmol of diphenylmethane into a two-necked bottle, add 20ml of double-distilled tetrahydrofuran under the protection of argon to dissolve, add 4.0ml of 2.5M / cyclohexane n-butyllithium under stirring in an ice bath, and stir for 30min;
[0050] Step 2, after stirring, 4mmol of 4,4' Dimethylbenzophenone was dissolved in 10ml redistilled tetrahydrofuran, added dropwise to the reaction system, and stirred at room temperature for 6h;
[0051] Step 3, after the reaction is finished, quench by adding saturated ammonium chloride, extract three times with dichloromethane, combine the organic phases, wash once with saturated brine, dry the organic phases with anhydrous sodium sulfate, and distill off the organic solvent under reduced pressure to obtain light yellow Liquid, dissolve the...
Embodiment 2
[0064] The preparation method of TS1 comprises the following steps:
[0065] (1) Synthesis of compound 1
[0066] Step 1: Add 13 mmol of diphenylmethane into a two-necked bottle, add 20 ml of distilled tetrahydrofuran under the protection of argon to dissolve, add 3.2 ml of 2.6M / cyclohexane n-butyllithium under stirring in an ice bath, and stir for 22 minutes;
[0067] Step 2, after the stirring is completed, dissolve 5 mmol of 4,4'-dimethylbenzophenone in 12 ml of redistilled tetrahydrofuran, add it dropwise to the reaction system, and stir at room temperature for 7 hours;
[0068] Step 3, after the reaction is finished, quench by adding saturated ammonium chloride, extract three times with dichloromethane, combine the organic phases, wash once with saturated brine, dry the organic phases with anhydrous sodium sulfate, and distill off the organic solvent under reduced pressure to obtain light yellow Liquid, dissolve the light yellow liquid with toluene, add 17mmol p-toluenes...
Embodiment 3
[0079] The preparation method of TS1 comprises the following steps:
[0080] (1) Synthesis of compound 1
[0081] Step 1: Add 15 mmol of diphenylmethane into a two-necked bottle, add 20 ml of redistilled tetrahydrofuran under the protection of argon to dissolve, add 3.8 ml of 2.9M / cyclohexane n-butyllithium under stirring in an ice bath, and stir for 20 to 30 minutes;
[0082] Step 2, after stirring, dissolve 5.5 mmol of 4,4'-dimethylbenzophenone in 18 ml of redistilled tetrahydrofuran, add it dropwise to the reaction system, and stir at room temperature for 9 h:
[0083] Step 3, after the reaction is finished, quench by adding saturated ammonium chloride, extract three times with dichloromethane, combine the organic phases, wash once with saturated brine, dry the organic phases with anhydrous sodium sulfate, and distill off the organic solvent under reduced pressure to obtain light yellow Liquid, dissolve the light yellow liquid with toluene, add 17.5mmol p-toluenesulfonic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com