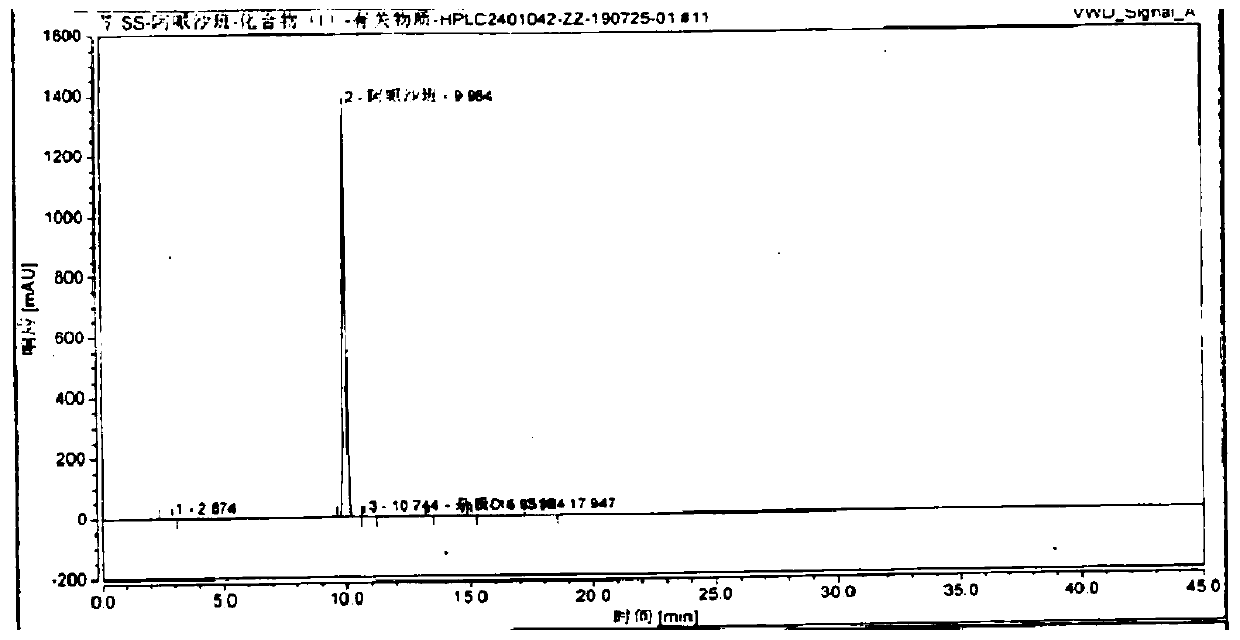
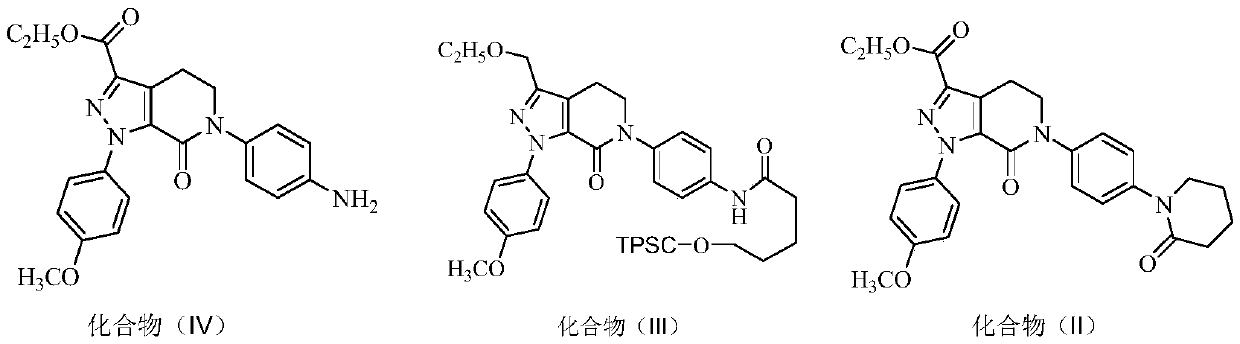
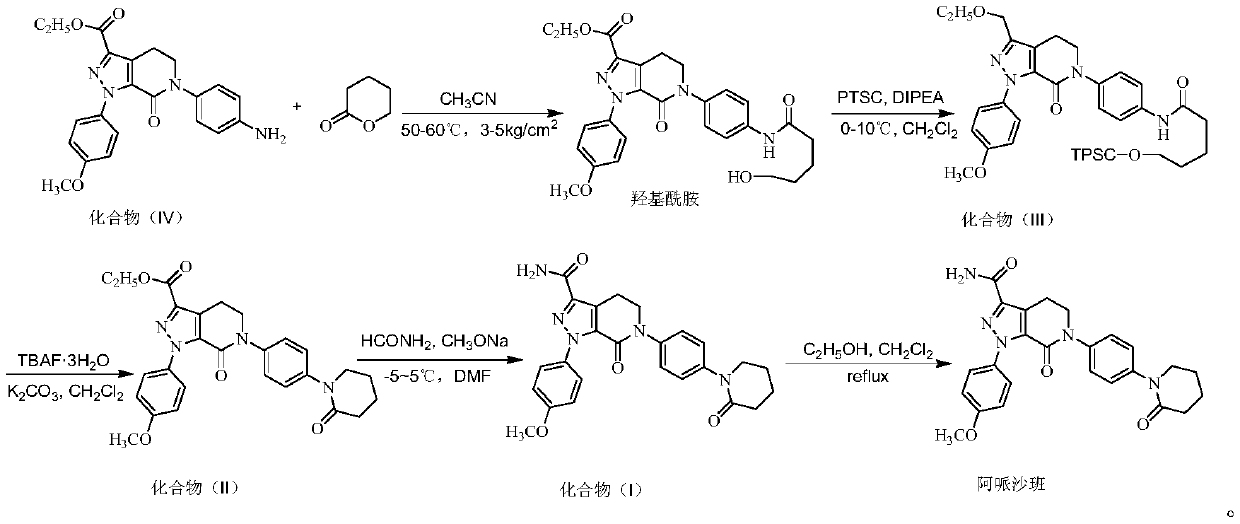
Preparation process of high-purity apixaban
A preparation process and technology of apixaban, applied in the field of preparation technology of high-purity apixaban
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A preparation process for high-purity apixaban, comprising the following steps:
[0051] S1: Preparation of Compound (Ⅲ):
[0052] Add 80mL of acetonitrile, 20g (49.3mmol) of compound (Ⅳ), 7.4g (73.9mmol) of δ-valerolactone successively in the pressure reactor, seal it, and feed nitrogen to make the pressure to 3kg / cm 2 , heat up to 50-60°C, keep stirring for 4-5 hours, lower the temperature, decompress and concentrate under reduced pressure to remove acetonitrile, add 150mL dichloromethane and 12g (93mmol) diisopropylethylamine after cooling down to room temperature, and cool down to 0 -10°C, add 12g (63mmol) p-toluenesulfonyl chloride dichloromethane solution dropwise, keep the reaction for 2 hours, wash with 100mL water and 100mL saturated sodium chloride solution successively, concentrate the washed organic layer under reduced pressure, add 140mL acetic acid The ethyl ester was stirred and heated to reflux, filtered after clarification, cooled to 0-10°C for crystal...
Embodiment 2
[0060] S1: Preparation of Compound (Ⅲ):
[0061] Add 80mL of acetonitrile, 20g (49.3mmol) of compound (Ⅳ), 7.4g (73.9mmol) of δ-valerolactone successively in the pressure reactor, seal it, and feed nitrogen to make the pressure to 3kg / cm 2 , heat up to 50-60°C, keep stirring for 4-5 hours, lower the temperature, decompress and concentrate under reduced pressure to remove acetonitrile, add 150mL dichloromethane and 12g (93mmol) diisopropylethylamine after cooling down to room temperature, and cool down to 0 -10°C, add 12g (63mmol) p-toluenesulfonyl chloride dichloromethane solution dropwise, keep the reaction for 2 hours, wash with 100mL water and 100mL saturated sodium chloride solution successively, concentrate the washed organic layer under reduced pressure, add 140mL acetic acid The ethyl ester was stirred and heated to reflux, filtered after clarification, cooled to 0-10°C to crystallize, filtered, and vacuum-dried to obtain 26.7 g of off-white or off-white solid compound ...
Embodiment 3
[0069] S1: Preparation of Compound (Ⅲ):
[0070] Add 80mL of acetonitrile, 20g (49.3mmol) of compound (Ⅳ), 7.4g (73.9mmol) of δ-valerolactone successively in the pressure reactor, seal it, and feed nitrogen to make the pressure to 3kg / cm 2 , heat up to 50-60°C, keep stirring for 4-5 hours, lower the temperature, decompress and concentrate under reduced pressure to remove acetonitrile, add 150mL dichloromethane and 12g (93mmol) diisopropylethylamine after cooling down to room temperature, and cool down to 0 -10°C, add 12g (63mmol) p-toluenesulfonyl chloride dichloromethane solution dropwise, keep the reaction for 2 hours, wash with 100mL water and 100mL saturated sodium chloride solution successively, concentrate the washed organic layer under reduced pressure, add 140mL acetic acid The ethyl ester was stirred and heated to reflux, filtered after clarification, cooled to 0-10°C for crystallization, filtered, and vacuum-dried to obtain 26.4 g of off-white or off-white solid comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com