Load type nano gold catalyst for preparing lactone by catalyzing air oxidation alpha, omega-diol and preparation method thereof
An air oxidation and catalyst technology, applied in the chemical industry, can solve the problems of dangerous operation, not in line with sustainable development and green chemistry, expensive and other problems, and achieve the effects of easy separation and recovery, good catalytic oxidation activity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
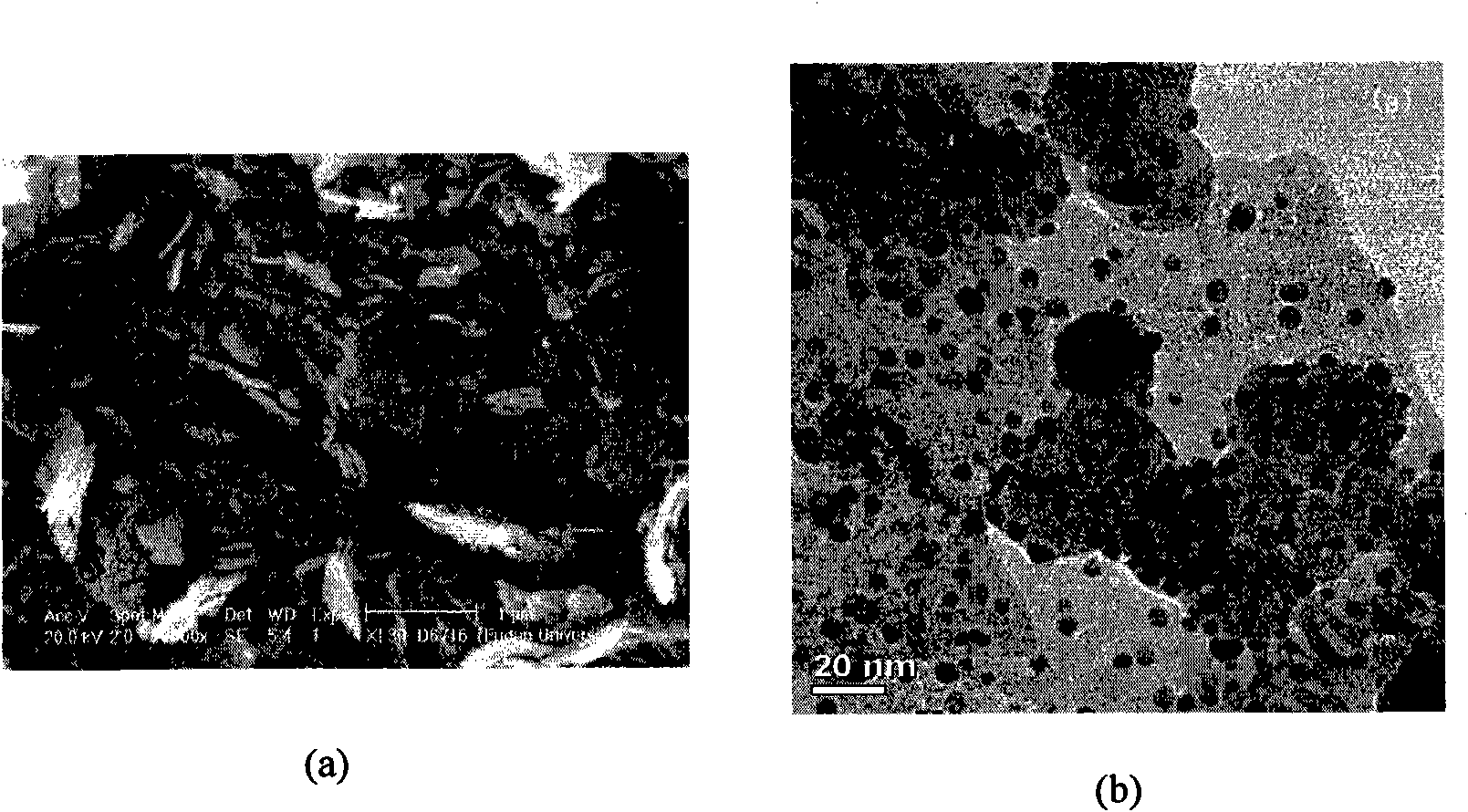
Image
Examples
Embodiment 1
[0022] 1.67g FeSO 4 ·7H 2 O was mixed with 50ml ethanolamine and dissolved by sonication. Then 50ml of deionized water was added, the resulting solution was transferred to a hydrothermal reaction kettle, and placed in an oven at 120° C. for 2 hours. The resulting mixture was centrifuged, washed with ethanol four times, dried, and calcined at 800° C. for 4 hours, and the obtained iron oxide carrier was 1# carrier. Add 10ml 2.43mmol / L HAuCl to 48ml deionized water 4 Solution, 2.91g urea and 0.6g 1# carrier, stirred at 60°C for 24h, suction filtered, washed, dried, and calcined at 100°C for 4 hours, the obtained catalyst was designated as 1# catalyst. Reaction conditions: 20ml tributyl phosphate, 1.4g 1,4-butanediol, 0.190g 1# catalyst, 1.25Mpa air, 100°C, electromagnetic stirring for 1 hour.
Embodiment 2
[0024] 1.67g FeSO 4 ·7H 2 O was mixed with 50ml ethanolamine and dissolved by sonication. Then 50ml of deionized water was added, the resulting solution was transferred to a hydrothermal reaction kettle, and placed in an oven at 70°C for 14h. The resulting mixture was centrifuged, washed with ethanol four times, dried, and then calcined at 300° C. for 4 hours, and the obtained iron oxide carrier was 2# carrier. Add 10ml 2.43mmol / L HAuCl to 48ml deionized water 4 Solution, 2.91g urea and 0.6g 2# carrier, stirred at 90°C for 1h, suction filtered, washed, dried, and calcined at 300°C for 4 hours, the obtained catalyst was designated as 2# catalyst. Reaction conditions: 20ml tributyl phosphate, 1.4g 1,4-butanediol, 0.190g 2# catalyst, 1.25Mpa air, 150°C, electromagnetic stirring for 2 hours.
Embodiment 3
[0026] 3.34 g FeSO 4 ·7H 2 O was mixed with 130ml ethanolamine and dissolved by sonication. Then 100ml of deionized water was added, and the resulting solution was transferred to a hydrothermal reaction kettle, and stood at 100°C for 5h. The obtained mixture was centrifuged, washed with ethanol four times, and dried at room temperature, and the obtained iron oxide carrier was 3# carrier. Add 10ml 2.43mmol / L HAuCl to 48ml deionized water 4 Solution, 2.91g urea and 0.6g 3# carrier, stirred at 80°C for 2h, suction filtered, washed, dried, and calcined at 500°C for 4 hours, the obtained catalyst was designated as 3# catalyst. Reaction conditions: 20ml tributyl phosphate, 1.4g 1,4-butanediol, 0.190g 3# catalyst, 1.25Mpa air, 50°C, electromagnetic stirring for 3 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com