Preparation method for 1-benzosuberone
A technology of benzocycloheptanone and catalyst, applied in the field of preparation of 1-benzocycloheptanone, can solve problems such as unfavorable environment, difficulty in obtaining benzobicyclic hydrocarbon compounds, harsh reaction conditions, etc., and achieves shortened process route, The effect of wide source of raw materials and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
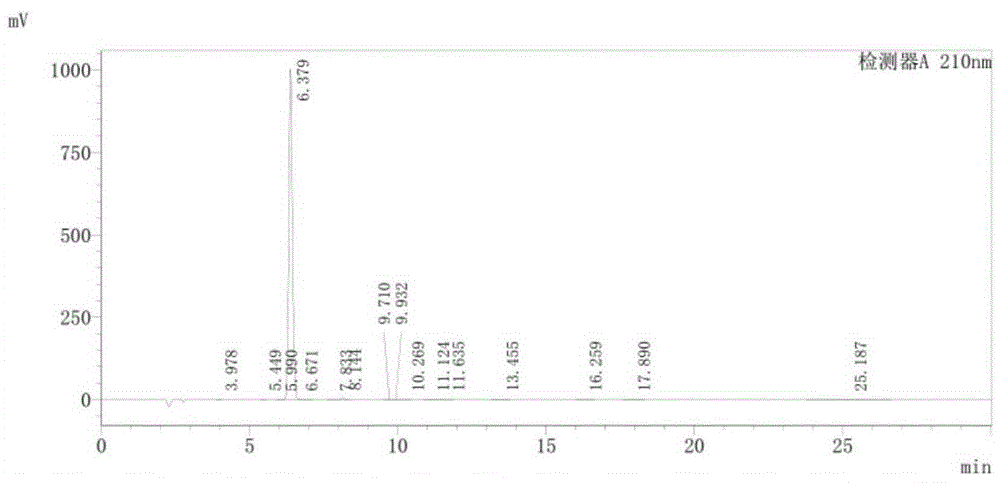
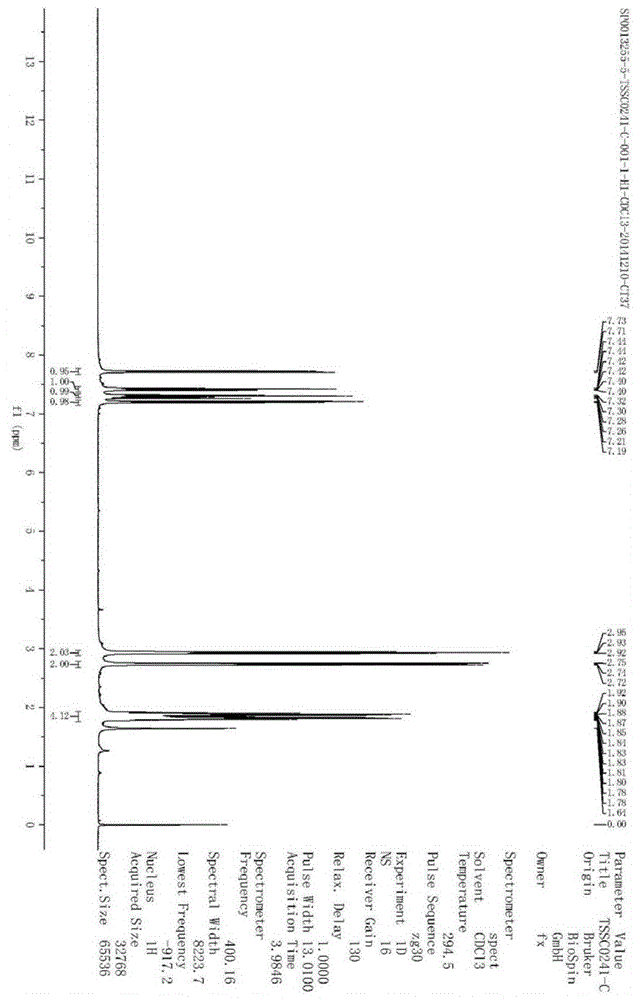
Image
Examples
Embodiment 1
[0037] The preparation method of 1-benzocycloheptanone is as follows:
[0038]Add 332.3g of delta-valerolactone and 2.33L of benzene in a 5L three-necked flask equipped with a thermometer, mechanical stirring, reflux pipe, and tail gas receiving device, start stirring, add 1.77kg of anhydrous aluminum trichloride in batches at room temperature, and control it at Complete the addition within 2 hours, heat the reaction system until the reflux reaction temperature is controlled at 80°C, and the reaction time is controlled at 14 hours. TLC detects that the δ-valerolactone reaction is complete, stop the reaction, cool to room temperature, and slowly pour the reaction system into 8kg crushed ice cubes and 1.4L of concentrated hydrochloric acid mixture, stirring, liquid separation. The aqueous phase was extracted once with 2L of toluene; the organic phases were combined, and the organic phase was washed with 10% NaOH solution and 1L of water successively, dried with sodium sulfate, f...
Embodiment 2
[0041] The preparation method of 1-benzocycloheptanone is as follows:
[0042] Add 332.3g of δ-valerolactone and 2.99L of benzene in a 5L three-necked flask equipped with a thermometer, mechanical stirring, reflux pipe, and tail gas receiving device, start stirring, add 1.892kg of anhydrous titanium tetrachloride in batches at room temperature, and control it at Complete the addition within 2 hours, heat the reaction system until the reflux reaction temperature is controlled at 90°C, and the reaction time is controlled at 16 hours. TLC detects that the δ-valerolactone reaction is complete, stop the reaction, cool to room temperature, and slowly pour the reaction system into 8kg crushed ice cubes and 1.4L of concentrated hydrochloric acid mixture, stirring, liquid separation. The aqueous phase was extracted once with 2L of ethyl acetate; the organic phases were combined, and the organic phase was washed with 10% KOH solution and 1L of water successively, dried with sodium sulfa...
Embodiment 3
[0044] The preparation method of 1-benzocycloheptanone is as follows:
[0045] Add 16.6g of δ-valerolactone and 132mL of benzene into a 250mL three-neck flask equipped with a thermometer, mechanical stirring, reflux tube, and tail gas receiving device, start stirring, add 83.9g of anhydrous aluminum trichloride in batches at room temperature, and control it within 30min Addition is complete; heat the reaction system to reflux, control the reaction at 85°C, and control the reaction time at 12h. TLC detects that the δ-valerolactone has completely reacted, stop the reaction, and cool to room temperature; slowly pour the reaction system into 400g crushed ice cubes and 70mL In a mixture of concentrated hydrochloric acid, stir and separate the liquids. The aqueous phase was extracted once with 200 mL of methyl tert-butyl ether; the organic phases were combined, and the organic phase was washed with 10% sodium carbonate solution and 200 mL of water successively, dried with sodium sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com