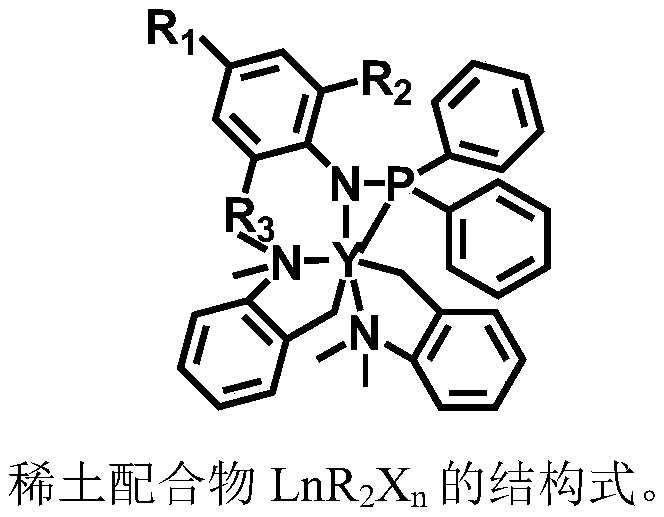
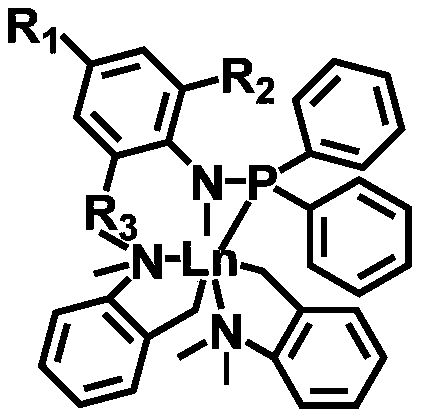
First-class rare earth macrolide/valerolactone/caprolactone terpolymer and preparation method thereof
A caprolactone terpolymer and caprolactone ternary technology, applied in the field of rare earth macrolide/valerolactone/caprolactone terpolymer and its preparation, to improve mechanical properties and thermal stability , mild reaction conditions and excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of embodiment 1 rare earth macrocyclic lactone / valerolactone / caprolactone terpolymer
[0016] Under nitrogen protection, 1.6 mmol of cycloethylene tridecanedioate, 1.2 mmol of δ-valerolactone and 1.2 mmol of ε-caprolactone were added to the reactor and mixed uniformly. Take 20μmol rare earth catalyst (C 6 h 5 NPPh 2 )Lu(CH 2 C 6 h 4 NMe 2 -o) 2 Dissolve in 1 mL of toluene and add to the mixture in the above reactor. The polymerization reaction temperature was 25°C, and the polymerization was carried out for 1000 minutes. Methanol was added to terminate the reaction, washed with methanol, and dried in vacuum to obtain a macrocyclic lactone / valerolactone / caprolactone terpolymer. The analysis results of polymer structure and properties are as follows: in mole percentage, the tridecanedioic acid cycloethylene ester content is 40%, the δ-valerolactone content is 30%, and the ε-caprolactone content is 30%. The number average molecular weight is 5.8×10...
Embodiment 2
[0017] Preparation of embodiment 2 rare earth macrocyclic lactone / valerolactone / caprolactone terpolymer
[0018] Under nitrogen protection, 2 mmol of cycloethylene tridecanedioate, 1.2 mmol of δ-valerolactone and 0.8 mmol of ε-caprolactone were added to the reactor and mixed uniformly. Take 20μmol rare earth catalyst (2-MeC 6 h 4 NPPh 2 )Sc(CH 2 C 6 h 4 NMe 2 -o) 2 Dissolve in 1 mL of toluene and add to the mixture in the above reactor. The polymerization reaction temperature was 25°C, and the polymerization was carried out for 1000 minutes. Methanol was added to terminate the reaction, washed with methanol, and dried in vacuum to obtain a macrocyclic lactone / valerolactone / caprolactone terpolymer. The polymer structure and property analysis results are as follows: in mole percentage, the tridecanedioic acid cycloethylene ester content is 50%, the δ-valerolactone content is 30%, and the ε-caprolactone content is 20%. The number average molecular weight is 7.2×10 4g / mo...
Embodiment 3
[0019] Preparation of embodiment 3 rare earth macrocyclic lactone / valerolactone / caprolactone terpolymer
[0020] Under nitrogen protection, 1.2 mmol of cycloethylene tridecanedioate, 1.2 mmol of δ-valerolactone and 1.6 mmol of ε-caprolactone were added to the reactor and mixed uniformly. Take 20μmol rare earth catalyst (4-MeC 6 h 4 NPPh 2 )Sc(CH 2 C 6 h 4 NMe 2 -o) 2 Dissolve in 1 mL of cyclohexane and add to the mixture in the above reactor. The polymerization reaction temperature was 25°C, and the polymerization was carried out for 1000 minutes. Methanol was added to terminate the reaction, washed with methanol, and dried in vacuum to obtain a macrocyclic lactone / valerolactone / caprolactone terpolymer. The polymer structure and property analysis results are as follows: in mole percentage, tridecanedioic acid cycloethylene ester content is 30%, δ-valerolactone content is 30%, ε-caprolactone content is 40%. The number average molecular weight is 5.9×10 4 g / mol, molecu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com