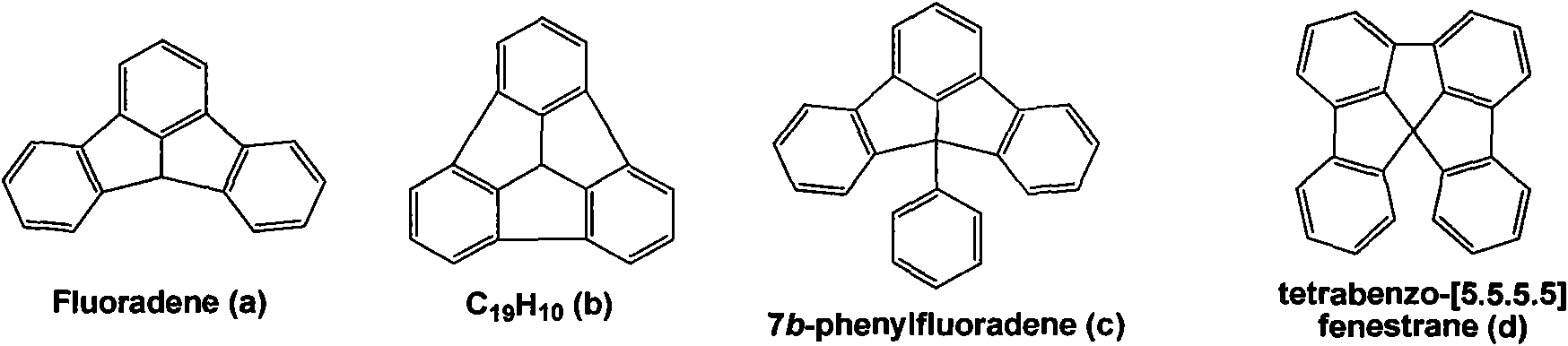
Fluoradene derivative and preparation method thereof
A technology of derivatives and compounds, applied in the field of Fluoradene derivatives and their preparation, can solve problems not involving synthesis details, etc., and achieve good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
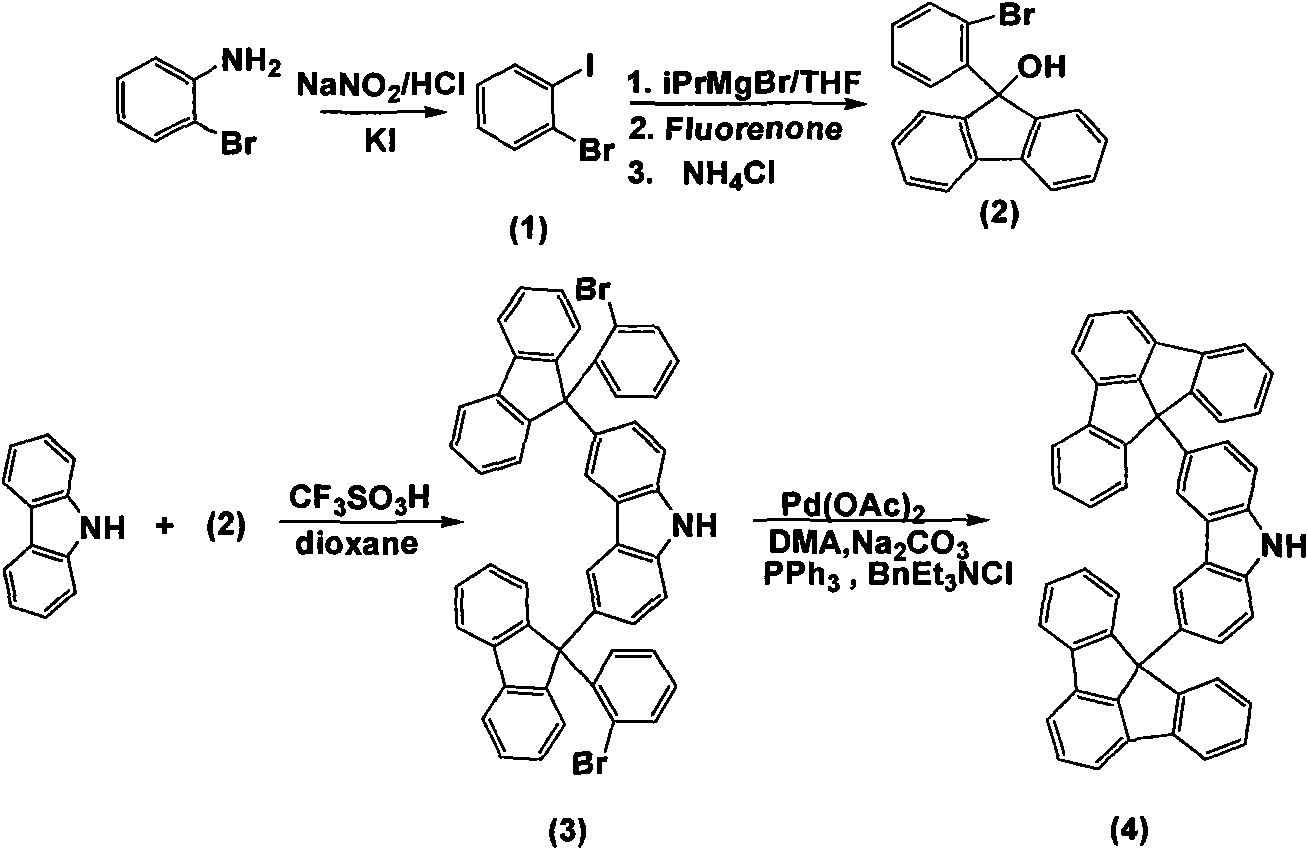
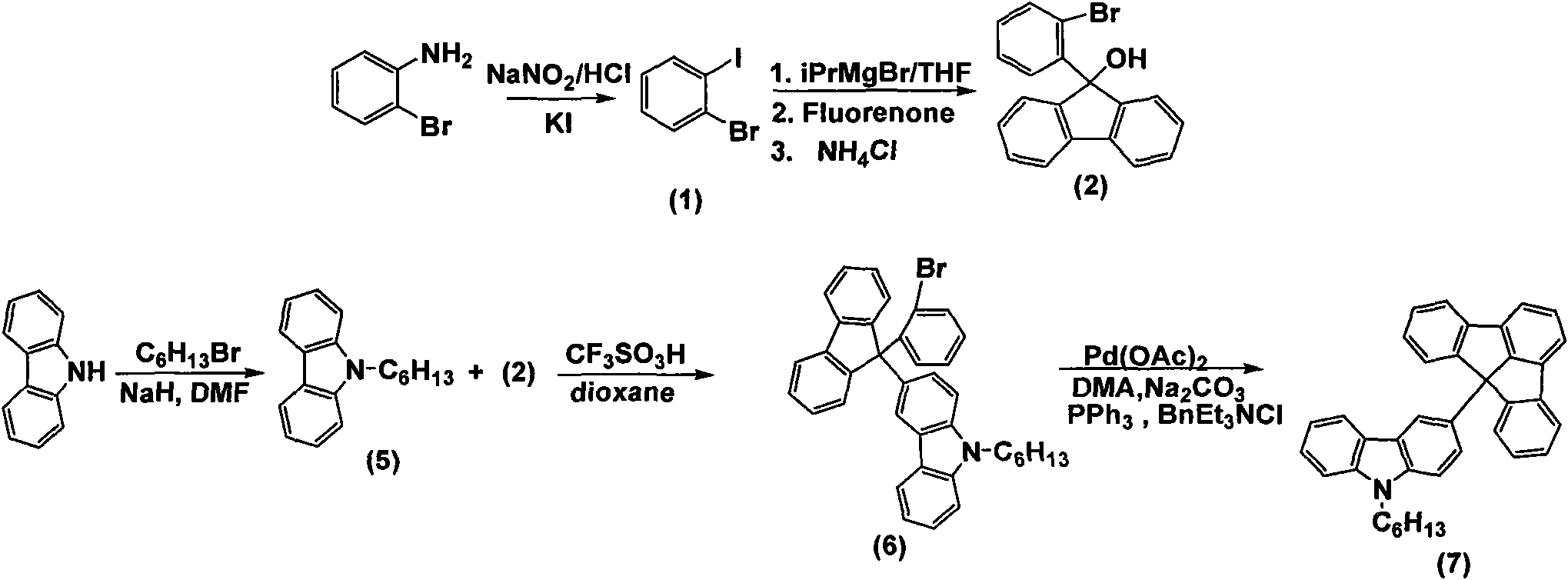
[0038] 1), the preparation of 2-bromoiodobenzene (1)
[0039] Dissolve o-bromoaniline (5g, 29.06mmol) in 50mL of water, then add 15mL of concentrated hydrochloric acid into it, and cool to 0°C. NaNO 2 (2.41g, 34.87mmol) was dissolved in 50mL of water and slowly added dropwise to the above solution. After all the solids were dissolved, KI (7.23 g, 43.59 mmol) dissolved in 11 mL of water was slowly added to the above solution, and the solution turned blood red. Stir at room temperature for 0.5h. Stir at 70°C for 0.5h. Cool, add 0.43mol / L Na 2 SO 3 Neutralize 20mL of the solution. Extracted with dichloromethane, the organic phase was washed with water, and then passed through anhydrous MgSO 4 dry. After suction filtration, the solvent was removed, and then distilled under reduced pressure to obtain a light yellow liquid (3.85 g, yield: 77.0%). 1 H NMR (CDCl 3 , 500MHz): δ7.88-7.86(dd, J=8.0Hz, J=1.4Hz 1H); 7.64-7.62(dd, J=8.0Hz, J=1.4Hz, 1H); 7.30-7.20(td, J =7.9Hz, J=...
Embodiment 2
[0050] 1) with (1) in embodiment 1
[0051] 2) with (2) in embodiment 1
[0052] 3) Preparation of 9-hexylcarbazole (5)
[0053] Under nitrogen protection, NaH (1.74g, 60%w / w dispersion in mineral oil, 44.0mmol) was poured into a round bottom flask containing 20mL N, N-dimethylacetamide, N 2 Protect. Carbazole (5.52 g, 33.0 mmol) was dissolved in 60 mL of N,N-dimethylacetamide and added dropwise to the above solution and stirred for 0.5 h. Add 1-bromohexane (6.54g, 39.6mmol) dropwise to it, and heat to reflux for 5h. Pour into water after cooling, extract with dichloromethane (3×50mL), and use MgSO for the organic phase 4 Dry, filter and spin dry. Recrystallization from ethanol gave a white solid (6.39 g, yield 77.0%). MP: 64-66°C
[0054] 4) Preparation of 3-(9-(2-bromophenyl)fluorenyl)-9-hexylcarbazole (6)
[0055]Under nitrogen protection, N-hexylcarbazole (2.80g, 11.12mmol) and trifluorosulfonic acid (0.67g, 4.45mmol) were dissolved in 10mL1,4-dioxane, and then 9-(...
Embodiment 3
[0063] 1) with (1) in embodiment 1
[0064] 2) with (2) in embodiment 1
[0065] 3) with (3) in embodiment 2
[0066] 4) Preparation of 3,6-bis[9-(2-bromophenyl)fluorenyl]-9-hexylcarbazole (8)
[0067] Under nitrogen protection, N-hexylcarbazole (1.29g, 5.19mmol) and 9-(2-bromophenyl)-9-fluorenol (3.5g, 10.37mmol) were dissolved in 50mL 1,4-dioxane , and then trifluorosulfonic acid (1.48g, 9.86mmol) was slowly dropped into the above solution, and then heated to 80°C for 5h. After cooling, the reaction solution was poured into water, filtered with suction, and dried. Through the column with petroleum ether:dichloromethane=4:1, a white solid (2.0 g, yield 43.3%) was obtained.
[0068] 1 H NMR (CDCl 3 500MHz): δ7.75(s, 4H); 7.69(br, 2H); 7.59(s, 2H); 7.51(d, J=7.0Hz, 4H); 7.40-7.36(m, 6H); 7.25(t , J=7.5Hz, 4H); 7.19(d, J=9.0Hz, 2H); 7.12-7.08(m, 4H); 7.06-7.03(m, 2H); 4.14(t, J=7.5Hz, 2H) ;1.76-1.72(m, 2H); 1.30-1.23(m, 6H); 0.83(t, J=7.0Hz, 3H).
[0069] 13 C NMR (CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com