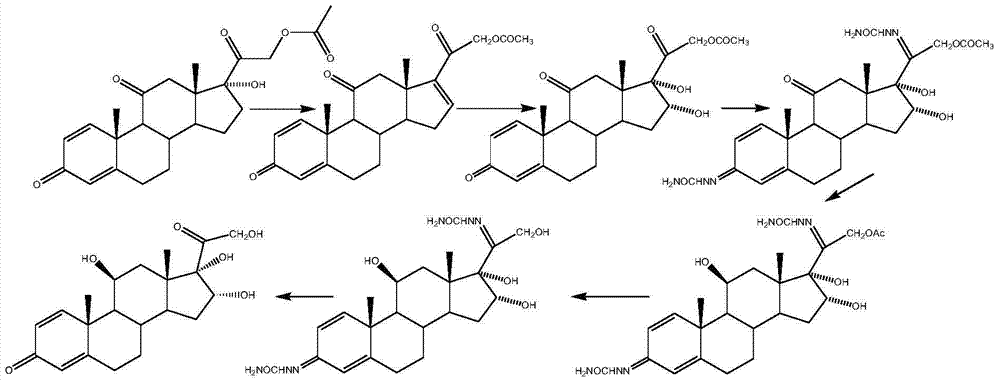
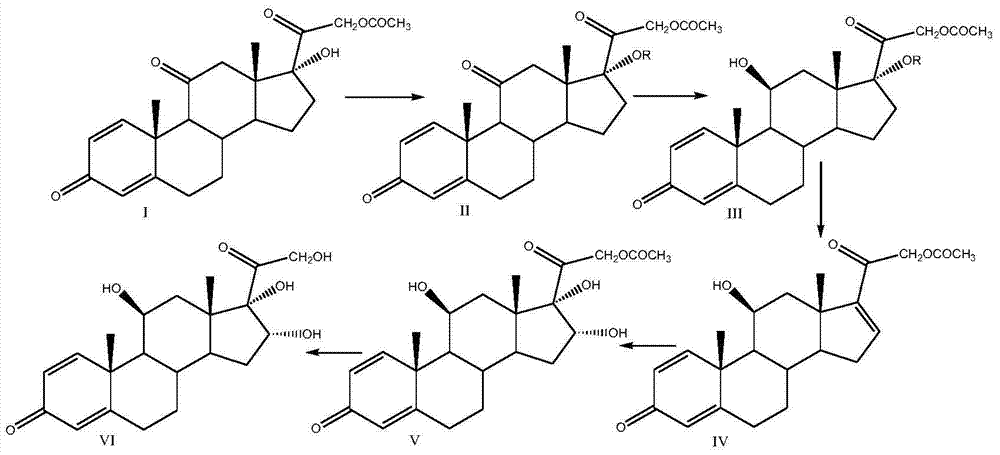
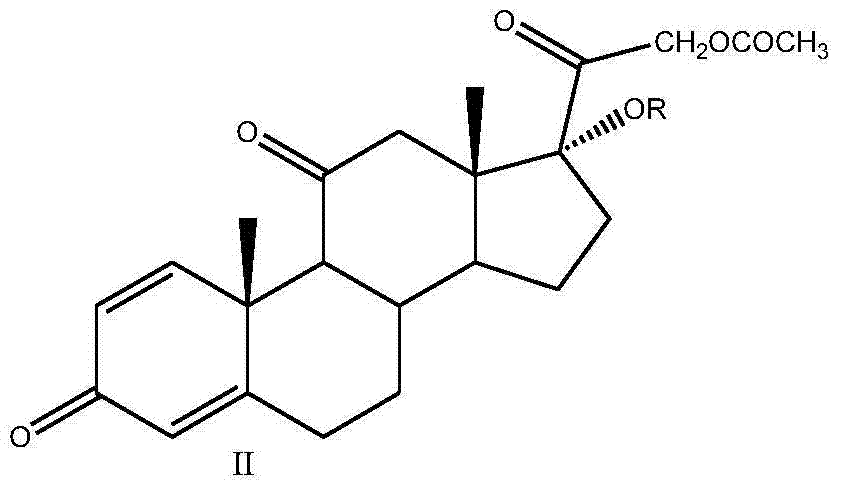
Preparation method of 16alpha-hydroxyprednisolone
A technology of hydroxyprednisolone and prednisone acetate, which is applied in the field of chemical synthesis of pharmaceutical intermediates, can solve the problems of difficulty in intermediate control, high production cost, and high equipment requirements, and achieves reduced side reactions, short reaction steps, and reduced The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Put 100g of prednisone acetate, 500ml of chloroform, 250ml of acetic anhydride and 5g of p-toluenesulfonic acid into the three-necked reaction flask, stir and heat up to reflux, keep it warm for 14-16h, after the reaction is completed, cool down to about 25°C, add 200ml of water dropwise Terminate the reaction, concentrate to dryness under reduced pressure, add 1000ml of water, stir for 30min, filter and wash with water until neutral, and dry to obtain 109g of compound II, namely 1,4,-diene-3,11,20-trione pregna-17α ,21-Diacetate; weight yield: about 109%, HPLC content: 99%.
[0030] Add 250ml of methanol, 250ml of dichloromethane, 50g of compound II, and 30g of anhydrous zinc chloride into the three-necked reaction flask, dissolve completely under stirring, cool down to about 10-15°C, and slowly add potassium borohydride to the reaction solution in batches The solid is 8g, after the reaction is completed, add glacial acetic acid to adjust the pH to 6~7, stir for 10 min...
Embodiment 2
[0035] Put 100g of prednisone acetate, 600ml of dichloroethane, 250ml of propionic anhydride and 4ml of concentrated sulfuric acid into the three-necked reaction bottle, stir and heat up to 80°C, keep it warm for 14-16h, after the reaction is complete, cool down to about 25°C, add 200ml of water dropwise to stop Reaction, concentrated under reduced pressure to gradually dryness, added 1000ml of water, stirred for 30min, filtered and washed with water until neutral, and dried to obtain 115g of compound II, namely 1,4,-diene-3,11,20-trione pregna-17α, -Propionate-21-acetate; weight yield: about 115%, HPLC content: 99%.
[0036] Add 350ml of ethanol, 250ml of chloroform, 50g of compound II, and 30g of anhydrous magnesium chloride into the three-necked reaction flask, dissolve completely under stirring, cool down to about 10-15°C, and slowly add potassium acetylborohydride to the reaction solution in batches The solid is 16g, after the reaction is completed, add glacial acetic aci...
Embodiment 3
[0041] Put 100g of prednisone acetate, 500ml of dichloromethane, 250ml of acetyl chloride and 100ml of pyridine into the three-necked reaction bottle, stir and heat up to 40°C, keep it warm for 2-4 hours, after the reaction is complete, cool down to about 25°C, add 100ml of water dropwise to terminate the reaction , concentrated to dryness under reduced pressure, added 1000ml of water, stirred for 30min, filtered and washed until neutral, and dried to obtain 109g of compound II, that is, 1,4,-diene-3,11,20-triketone pregna-17α,21 - diacetate; weight yield: about 109%, HPLC content: 99%.
[0042] Add 250ml of anhydrous methanol, 250ml of dichloromethane, 50g of compound II, and 30g of anhydrous nickel chloride into the three-necked reaction flask, dissolve completely under stirring, cool down to about -10~-5°C, and slowly pour into the reaction solution in batches Add 4g of solid lithium aluminum hydride, after the reaction is complete, add glacial acetic acid to adjust the pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com