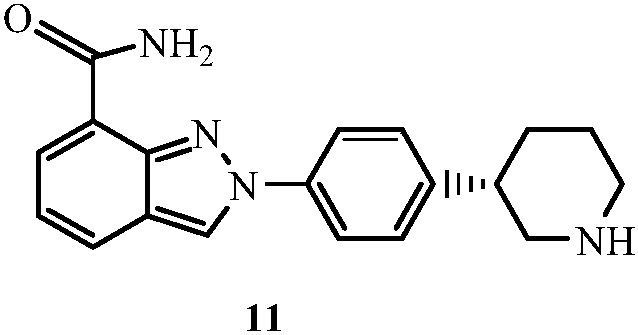
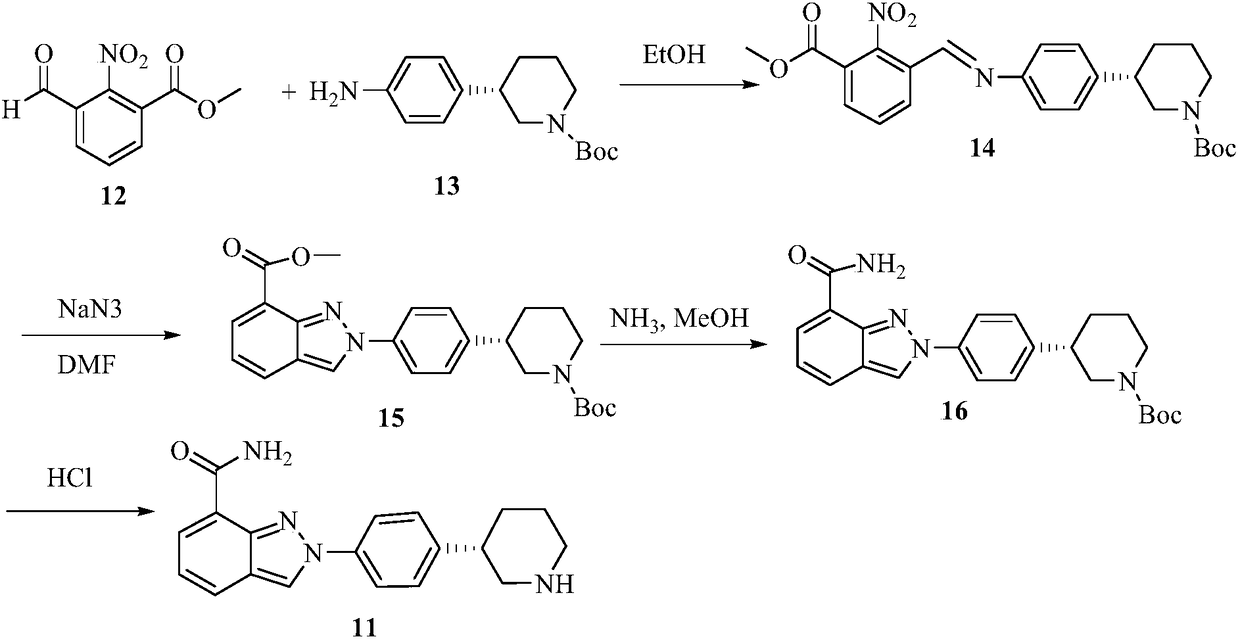
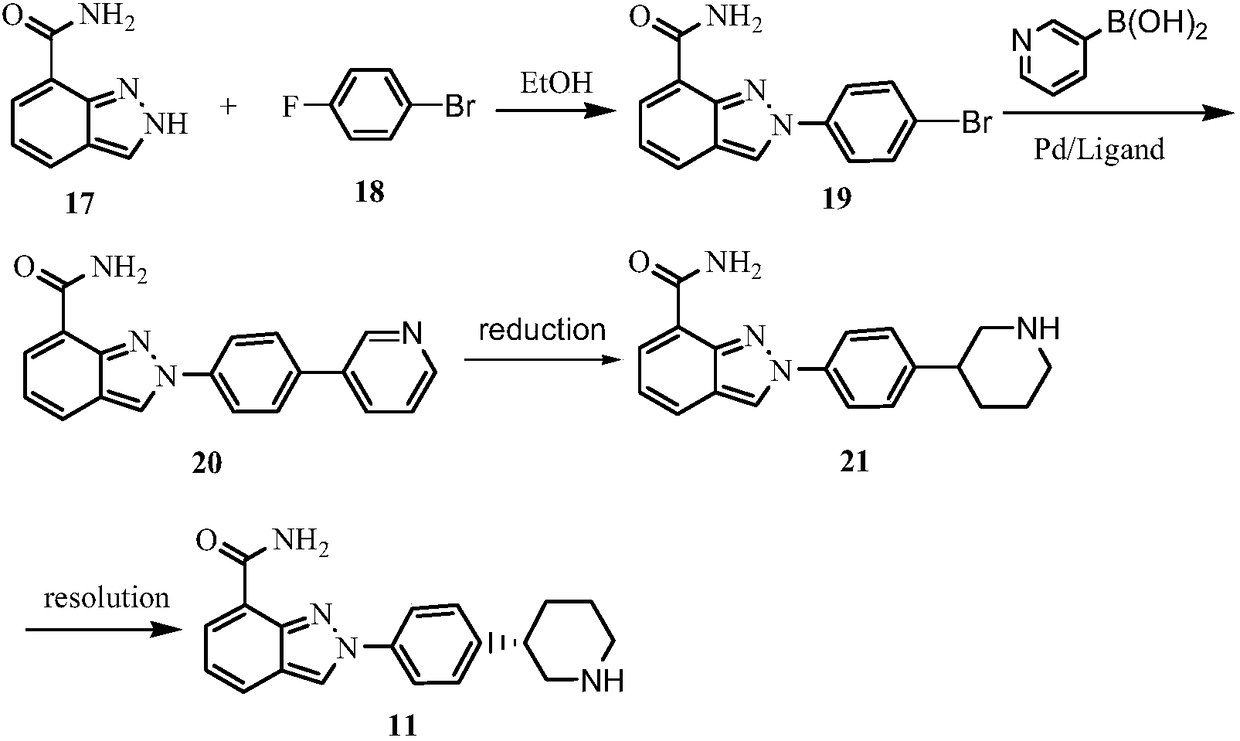
Synthesis method for (R)-3-phenylpiperidine or/and (S)-3-phenylpiperidine and synthesis method for chiral intermediate of niraparib
A chiral intermediate, phenylpiperidine technology, applied in the field of synthesis of chiral intermediates, can solve the problems of high reaction yield, high synthesis process cost, mild reaction conditions, etc., and achieves high reaction yield and reduced production. Cost, mild effect of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: Synthesis of 3-hydroxyl-3-phenyl-1-benzylpiperidine (3)
[0076] Scheme 10:
[0077]
[0078] Phenylmagnesium bromide Grignard reagent (2mol / L in THF, 240mL) and anhydrous tetrahydrofuran (300mL) were added to the reaction flask, protected by nitrogen, cooled to 0°C in an ice-water bath, and N-benzyl-3- Piperidone (2,60.0g, 317.0mmol) was diluted with anhydrous tetrahydrofuran (300mL), added to the dropping funnel, slowly added dropwise to the reaction flask, and the temperature was controlled at 0-5°C. After completion, continue to stir and react for 1 hour. TLC detects that the raw materials have reacted completely. Add saturated ammonium chloride aqueous solution (300mL) while stirring, and then extract with ethyl acetate (200mL×3). After three extractions, combine the organic layers and add an appropriate amount of Dry over sodium sulfate, filter, and spin dry the solvent to obtain 76.5 g of the crude product (3-hydroxy-3-phenyl-1-benzylpiperidine ...
Embodiment 2
[0079] Embodiment 2: Synthesis of 3-hydroxyl-3-phenyl-1-benzylpiperidine (3)
[0080] Scheme 11:
[0081]
[0082] Phenylmagnesium bromide Grignard reagent (2mol / L in THF, 240mL) and anhydrous ether (300mL) were added to the reaction flask, protected by nitrogen, cooled to 0°C in an ice-water bath, and N-benzyl-3- Piperidone (2,60.0g, 317.0mmol) was diluted with anhydrous diethyl ether (300mL), added to the dropping funnel, slowly added dropwise to the reaction flask, and the temperature was controlled at 0-5°C. After 60min, dropwise added After completion, continue to stir and react for 1 hour. TLC detects that the raw materials have reacted completely. Add saturated ammonium chloride aqueous solution (300mL) under stirring, and then extract with ethyl acetate (200mL×3). After extracting three times, combine the organic layers, and then add An appropriate amount of anhydrous sodium sulfate was added to the organic layer to dry, filtered, and the solvent was spin-dried to ...
Embodiment 3
[0083] Embodiment 3: Synthesis of 3-hydroxyl-3-phenyl-1-benzylpiperidine (3)
[0084] Scheme 12:
[0085]
[0086] Phenylmagnesium chloride Grignard reagent (2mol / L in THF, 240mL) and anhydrous tetrahydrofuran (300mL) were added to the reaction flask, protected by nitrogen, cooled to 0°C in an ice-water bath, and N-benzyl-3-piperidine Ketone (2,60.0g, 317.0mmol) was diluted with anhydrous tetrahydrofuran (300mL), added to the dropping funnel, and slowly added dropwise to the reaction flask, the temperature was controlled at 0-5°C, and the drop was completed after 50 minutes. Continue to stir and react for 1.5 hours, TLC detects that the raw materials have reacted completely, add saturated ammonium chloride aqueous solution (300mL) under stirring, and then extract with ethyl acetate (200mL×3), after extracting three times, combine the organic layers, and then add to the combined organic An appropriate amount of anhydrous sodium sulfate was added to the layer to dry, filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com