Method for preparing semaglutide
The technology of semaglutide and resin is applied in the field of preparation of semaglutide, which can solve the problems of decreased economic benefit, decreased yield, side reactions, etc., so as to reduce the risk of His racemization, reduce the coupling synthesis steps, Reduce the effect of increasing the number of couplings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
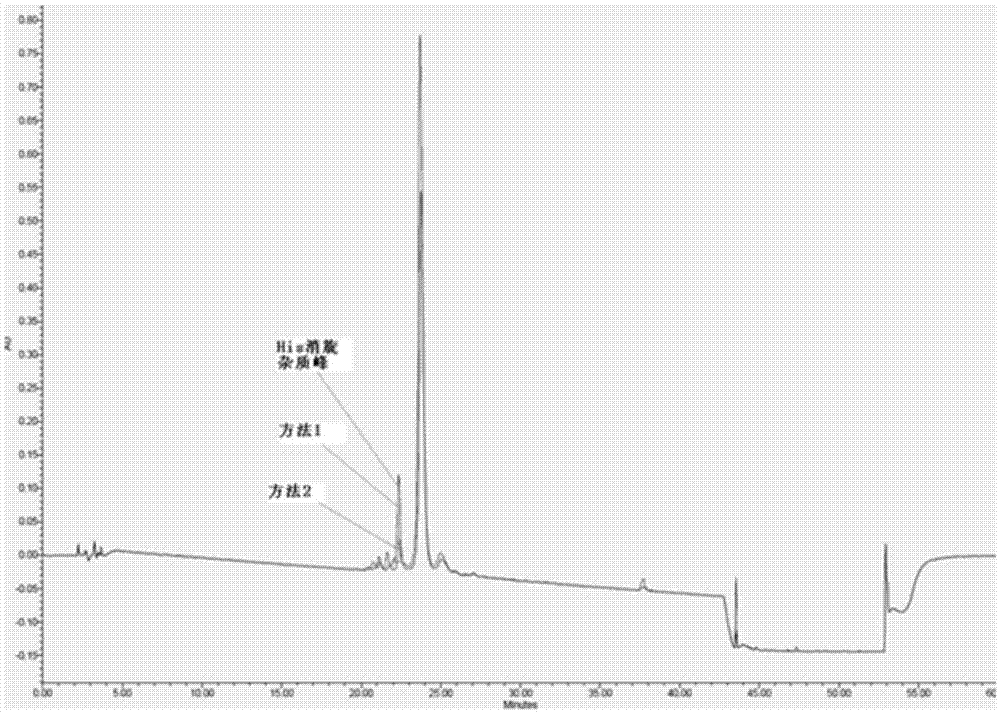
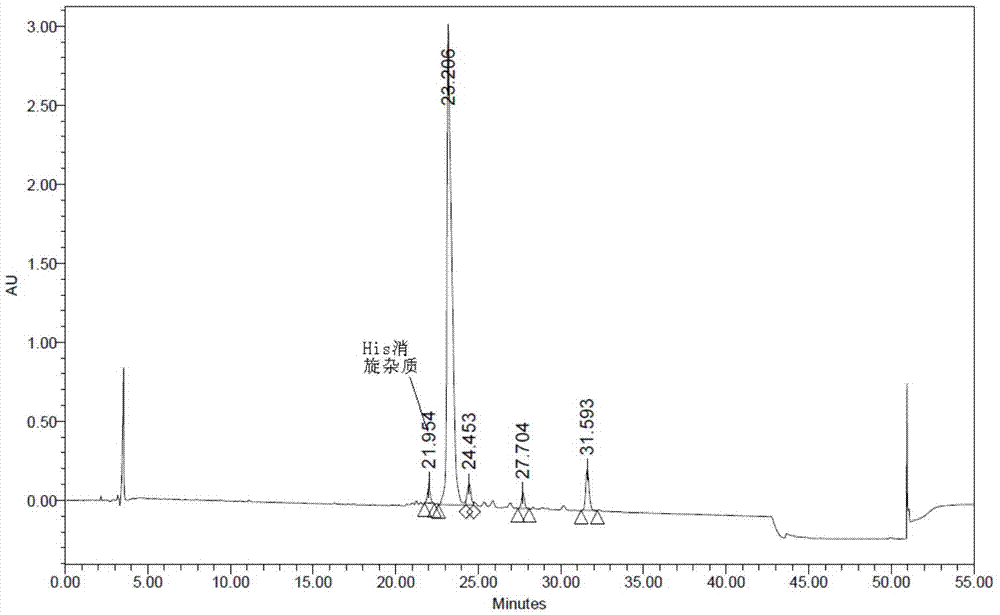
[0049] The invention discloses a preparation method of somaglutide, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all deemed to be included in the present invention. The method and application of the present invention have been described through the preferred embodiments. It is obvious that relevant persons can modify or appropriately change and combine the methods and applications described herein without departing from the content, spirit and scope of the present invention to achieve and Apply the technology of the present invention.
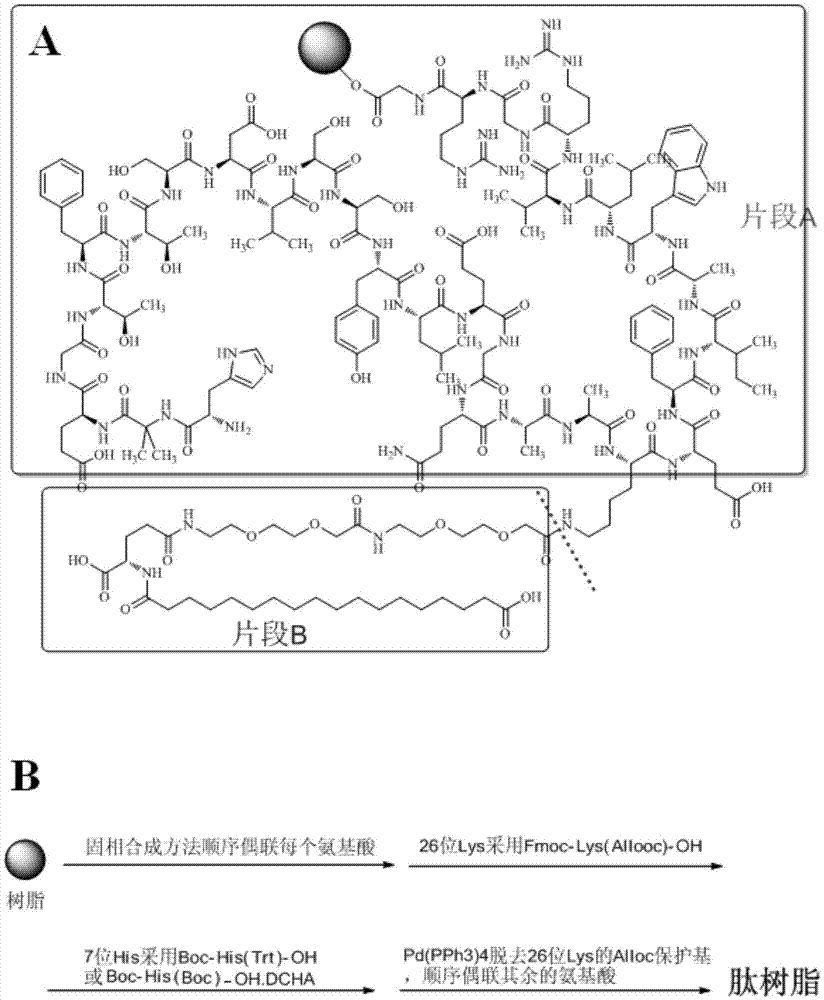
[0050] The present invention describes two methods for synthesizing somaglutide:
[0051] Method 1: Sequential coupling selection
[0052] Sequential coupling route map as figure 1 (B) shows:
[0053] Method 2: Sequential...
Embodiment 1
[0078] Example 1: Preparation of Fmoc-Gly-Wang Resin
[0079] Weigh 210g of dry Wang Resin (with a degree of substitution of 0.845mmol / g) into the solid phase reaction column, first wash the resin twice with DMF, then swell the resin with 2 to 3 times the volume of the resin bed for 30 minutes, and wash 3 times with DMF , DCM wash 2 times, waiting for feeding.
[0080] Under ice-cooling conditions, dissolve 105.48g Fmoc-Gly-OH and 47.92g HOBt in a mixed solvent of DMF and DCM. After the amino acid is dissolved, slowly add 55ml DIC, activate for 3min, and pour the reaction solution into the reaction. In the column, add 2.17g of DMAP, agitate and react for 2 hours; after the reaction, after washing with DMF 3 times, add an appropriate amount of acetic anhydride and pyridine mixed solution (volume ratio: Ac2O / Pyridine=7 / 6) to block the reaction for 5 hours Or above, after the reaction is stopped, DMF is washed 6 times, methanol is shrunk twice, and dried under reduced pressure to obt...
Embodiment 2
[0081] Example 2: Preparation of Fmoc-Gly-Wang Resin
[0082] Weigh 218g of dry Wang Resin (substitution degree is 0.845mmol / g) into the solid phase reaction column, first wash the resin with DMF 2 times, then swell the resin with 2 to 3 times the resin bed volume DMF for 30 minutes, and wash 3 times with DMF , DCM wash 2 times, waiting for feeding.
[0083] Under the condition of ice bath cooling, 26.88g Fmoc-Gly-OH and 12.03g HOBt are dissolved in a mixed solvent of DMF and DCM. After the amino acid is dissolved, slowly add 11ml DIC, activate for 3min, and pour the reaction solution into the reaction. In the column, add 1.12g of DMAP, stir the reaction for 45min with aeration; after the reaction, after washing with DMF for 3 times, add an appropriate amount of acetic anhydride and pyridine mixed solution (volume ratio: Ac2O / Pyridine=7 / 6) to block the reaction overnight, and react After stopping, DMF was washed 6 times, methanol was shrunk twice, and dried under reduced pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com