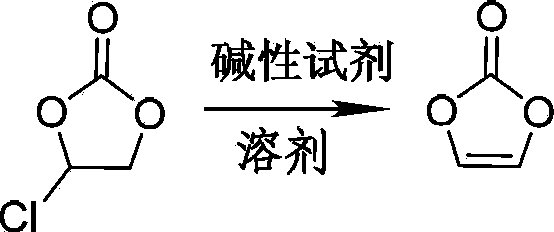
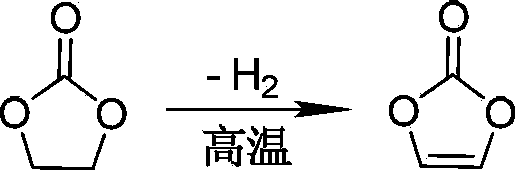
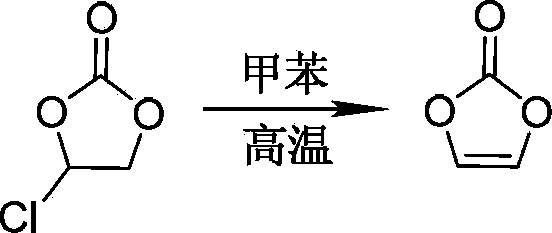
Preparation method of vinylene carbonate
A technology of vinylene carbonate and chloroethylene carbonate, applied in the field of preparing vinylene carbonate, can solve the problem of low yield of target product vinylene carbonate, low flash point of ether compounds, difficulty in purifying vinylene carbonate, etc. It is beneficial to large-scale industrial production, accelerates the reaction speed, and is easy to separate and purify.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A 250mL there-necked flask equipped with a stirring device was flushed with nitrogen, and 0.2 mol of dry ethylene monochlorocarbonate was added to the there-necked flask under nitrogen protection, and the temperature of the monochloroethylene carbonate in the there-necked flask was controlled at 40°C; Under stirring, 0.3 mol of redistilled triethylamine was added dropwise to ethylene monochlorocarbonate within 15 minutes through a dropping funnel. After the dropwise addition of triethylamine, the temperature was raised to 70°C, and the temperature was kept for 120 minutes. . The reaction was terminated to obtain a reaction solution containing vinylene carbonate.
[0029] The obtained reaction solution containing vinylene carbonate was filtered while hot, and the filter residue was washed three times with ethyl acetate. The filtrate was collected, and ethyl acetate and triethylamine were removed by rotary evaporator under the conditions of bath temperature of 55° C. and...
Embodiment 2
[0041] A 250mL three-necked flask equipped with a stirring device was flushed with nitrogen, and 0.2 mol of dry ethylene monochlorocarbonate (purity greater than 90%) was added to the three-necked flask under nitrogen protection to control the amount of monochloroethylene carbonate in the three-necked flask. The temperature is 43 ° C; under stirring, 1.0 mol of redistilled triethylamine is added dropwise to ethylene monochlorocarbonate within 45 minutes through a dropping funnel, and the temperature is raised to 50 °C after the triethylamine is added dropwise. °C and hold for 20 minutes. The reaction was terminated to obtain a reaction solution containing vinylene carbonate.
[0042] The obtained reaction solution containing vinylene carbonate was filtered while hot, and the filter residue was washed three times with ethyl acetate. The filtrate was collected, and ethyl acetate and triethylamine were removed by rotary evaporator under the conditions of bath temperature of 50° ...
Embodiment 3
[0046] A 250mL three-necked flask equipped with a stirring device was flushed with argon, and 0.2 mol of dry ethylene monochlorocarbonate (purity greater than 90%) was added to the three-necked flask under argon protection to control the amount of monochlorocarbonic acid in the three-necked flask. The temperature of vinyl ester is 45°C; under stirring, 0.6 mol of redistilled triethylamine is added dropwise to ethylene monochlorocarbonate within 30 minutes through a dropping funnel, and the temperature is raised after the addition of triethylamine is completed. to 60°C and held for 40 minutes. The reaction was terminated to obtain a reaction solution containing vinylene carbonate.
[0047] The obtained reaction solution containing vinylene carbonate was filtered while hot, and the filter residue was washed three times with ethyl acetate. The filtrate was collected, and ethyl acetate and triethylamine were removed by rotary evaporator under the conditions of bath temperature of 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com