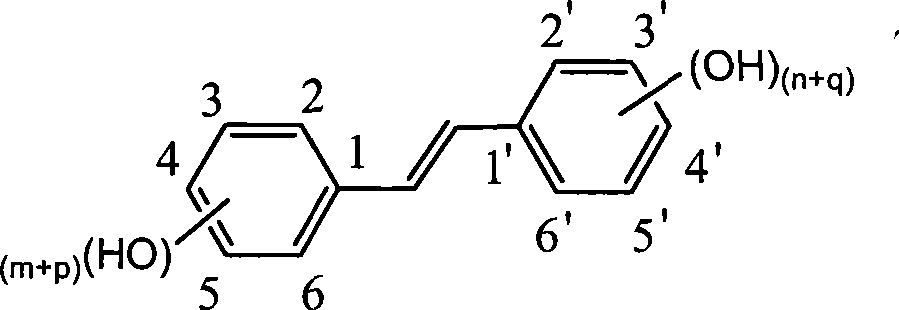
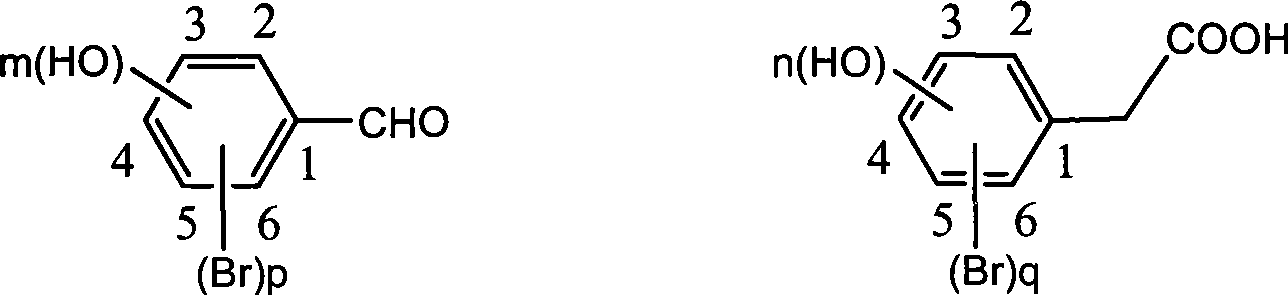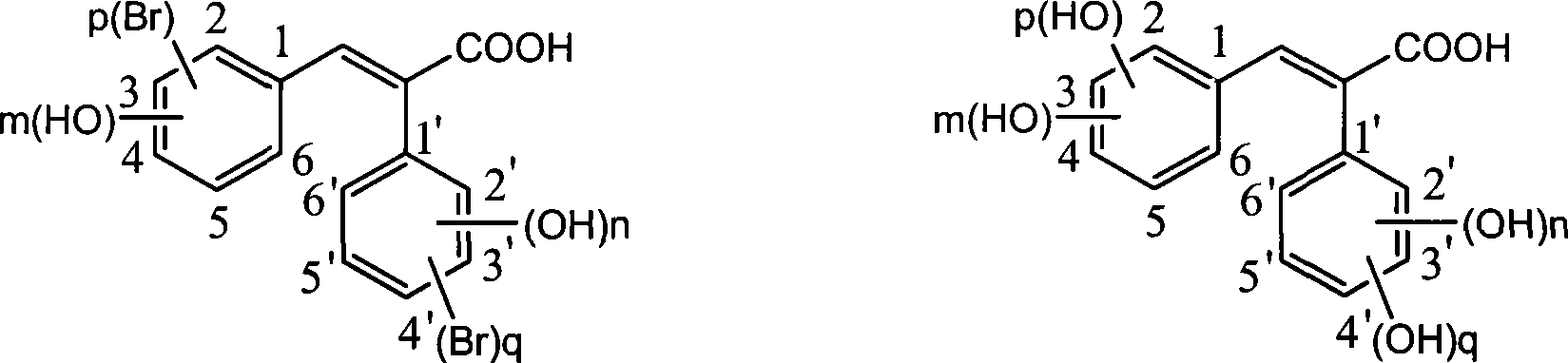Preparation of trans-polyhydroxy diphenyl ethylene
A technology of polyhydroxy stilbene and hydroxyphenylacetic acid, which is applied in the field of preparation of trans polyhydroxy stilbene, can solve the problems that hydroxyl groups need to be protected and deprotected, the reaction selectivity is not high, and industrialization is difficult, and the cost can be achieved. Low cost, good atom economy, and easy purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 5.88g (0.02mol) of 3,5-dibromophenylacetic acid, add 2.44g (0.02mol) of p-hydroxybenzaldehyde into the reaction flask, dissolve with 8.16g (0.08mol) of acetic anhydride, then add 5.05g (0.05mol) ) triethylamine, heated to 110°C, reacted for 6 hours, poured into ice water to precipitate solid, dissolved with 10% sodium hydroxide, washed with ethyl acetate, and crystallized at low temperature after acidifying the water layer to obtain light yellow solid (E) -2-(3',5'-dibromophenyl)-3-(4'-hydroxyphenyl)-acrylic acid, weighed dry to obtain 7.28g, yield 91.5%, recrystallized with ethanol-water to obtain 6.51g White crystals, yield 81.8%.
Embodiment 2
[0034] Weigh 5.88g (0.02mol) of 3,5-dibromophenylacetic acid, add 1.95g (0.016mol) of p-hydroxybenzaldehyde into the reaction flask, dissolve with 4.08g (0.04mol) of acetic anhydride, and then add 2.02g (0.02mol) ) triethylamine, heated to 100°C, reacted for 8 hours, poured into ice water to precipitate solid, dissolved with 10% sodium hydroxide, washed with ethyl acetate, and crystallized at low temperature after acidifying the water layer to obtain light yellow solid (E) -2-(3',5'-dibromophenyl)-3-(4'-hydroxyphenyl)-acrylic acid, weighed dry to obtain 5.49g, yield 86.2%, recrystallized from methanol-water to obtain 4.55g White crystals, yield 71.5%.
Embodiment 3
[0036] Weigh 5.88g (0.02mol) of 3,5-dibromophenylacetic acid, add 2.93g (0.024mol) of p-hydroxybenzaldehyde into the reaction flask, dissolve with 12.24g (0.12mol) of acetic anhydride, then add 8.08g (0.08mol) ) triethylamine, heated to 140°C, reacted for 2 hours, poured into ice water to precipitate solid, dissolved with 10% sodium hydroxide, washed with ethyl acetate, and crystallized at low temperature after acidifying the water layer to obtain light yellow solid (E) -2-(3',5'-dibromophenyl)-3-(4'-hydroxyphenyl)-acrylic acid, weighed dry to obtain 7.02g, yield 88.2%, recrystallized from ethyl acetate-petroleum ether 4.31 g of white crystals were obtained, with a yield of 79.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com