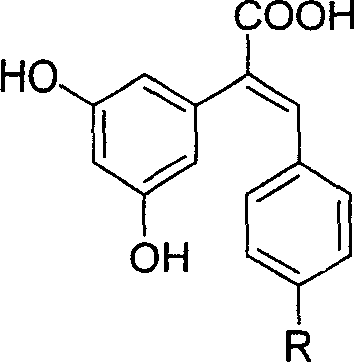
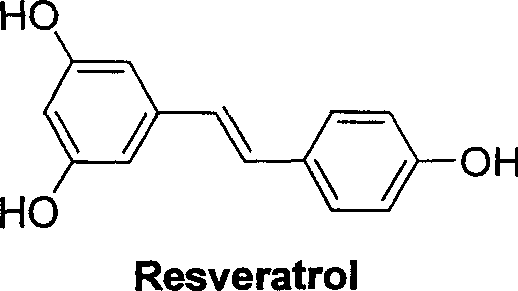
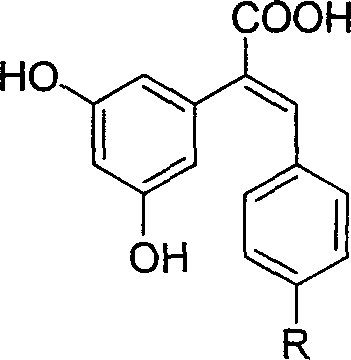
Carboxyl substituted resveratrol analog compound and its preparation method
A technology of resveratrol and compound, applied in the directions of nitrile preparation, organic chemistry, drug combination, etc., can solve problems such as cell inability to complete transformation, reduction of cyclin protein expression, G1 phase arrest of epidermal cancer A431 cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of (E)-2-(3,5-dihydroxyphenyl)-3-(4-hydroxyphenyl)acrylic acid
[0043] Synthesis of 3,5-dimethoxyphenylacetonitrile (4)
[0044] Solid sodium cyanide (14.3 g, 0.29 mol) was dissolved in distilled water (80 mL). Add absolute ethanol (80mL) into a 250mL three-necked flask, slowly add sodium cyanide solution under stirring, after the addition is complete, raise the temperature to 65°C, add 3,5-dimethoxybenzyl bromide (60g, 0.26mol), and complete the addition Reaction at 65°C for 1.5h. After the reaction, ethanol was evaporated under reduced pressure, cooled to room temperature, the precipitated solid was filtered, washed with water, recrystallized with methanol / water volume ratio 1 / 1, and dried to obtain the product, 44g of colorless needle crystals. Yield 96%, m.p.52-53°C.
[0045] Synthesis of 3,5-dimethoxyphenylacetic acid (3)
[0046] In a 150 ml three-neck flask, 17.7 g (0.1 mol) of 3,5-dimethoxyphenylacetonitrile (4) and 50 ml of metha...
Embodiment 2
[0051] Embodiment 2: the synthesis of (E)-2-(3,5-dihydroxyphenyl)-3-phenylacrylic acid
[0052] Synthesis of (E)-2-(3,5-dimethoxyphenyl)-3-phenylacrylic acid (2b)
[0053] Using similar reaction conditions as in Example 1, react 1.96 g of 3,5-dimethoxyphenylacetic acid (3) with 1.1 g of benzaldehyde, stir and heat up to 40° C. until the solid dissolves, add 0.7 g of potassium carbonate, and react for 2.5 hours 1.7 g of (E)-2-(3,5-dimethoxyphenyl)-3-phenylacrylic acid (2b) was obtained as a white powder solid, with a yield of 60% and a melting point of 203-205°C.
[0054] Synthesis of (E)-2-(3,5-dihydroxyphenyl)-3-phenylacrylic acid (1b)
[0055] Using the similar reaction conditions of Example 1, by 5.7g (2b) and 50mL, 2mol / L BBr 3 CH 2 Cl 2 The solution was reacted in 150 mL of dry dichloromethane, reacted at room temperature for 30 h, the reaction product was precipitated in ice water, filtered, and the solid was recrystallized with ethanol / water volume ratio 1 / 1 to obta...
Embodiment 3
[0056] Embodiment 3: Synthesis of (E)-2-(3,5-dihydroxyphenyl)-3-(4-nitrophenyl)acrylic acid
[0057] Synthesis of (E)-2-(3,5-dimethoxyphenyl)-3-(4-nitrophenyl)acrylic acid (2c)
[0058] Using similar reaction conditions as in Example 1, 1.96 grams of 3,5-dimethoxyphenylacetic acid (3) was reacted with 1.5 grams of p-nitrobenzaldehyde, stirred and heated to 40° C. until the solid was dissolved, and 0.7 grams of potassium carbonate was added. After reacting for 2.5 hours, 2.2 g of yellow needle-like crystal (E)-2-(3,5-dimethoxyphenyl)-3-(4-nitrophenyl)acrylic acid (2c) was obtained, with a yield of 67%. The melting point is 203-205°C.
[0059] Synthesis of (E)-2-(3,5-dihydroxyphenyl)-3-(4-nitrophenyl)acrylic acid (1c)
[0060] Using the similar reaction conditions of Example 1, by 6.6g (2c) and 50mL, 2mol / L BBr 3 CH 2 Cl 2 The solution was reacted in 150 mL of dry dichloromethane, reacted at room temperature for 24 hours, the reaction product was precipitated in ice water, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com