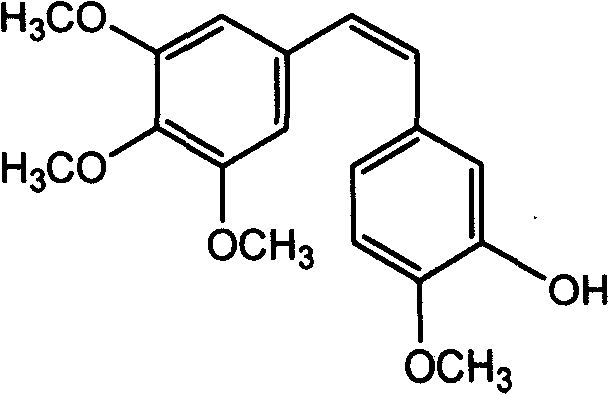
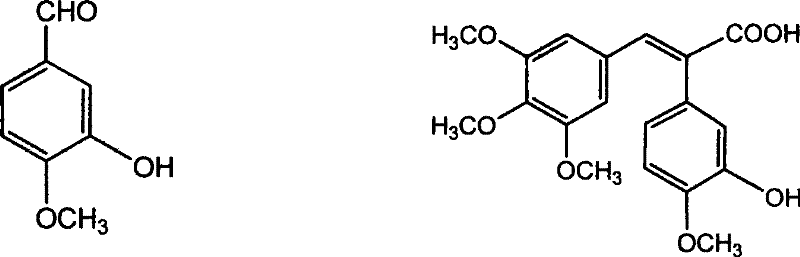
Method of preparing (Z)-3'-hydroxyl-3,4,4',5-tetramethoxy toluylene
A technology of tetramethoxystilbene and trimethoxybenzene is applied in the field of medicine and chemical industry to achieve the effects of improving cis-selectivity, simple post-processing and reducing synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
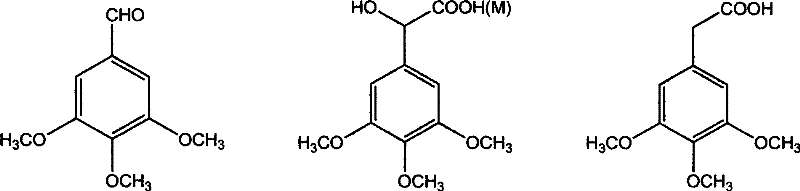
[0039] Add 3,4,5-trimethoxybenzaldehyde 3.92g (0.02mol), 0.5g tetrabutylammonium bromide (TBAB) and 25ml chloroform to a 100ml three-necked flask equipped with a thermometer, reflux condenser and dropping funnel , be warming up to 60 ℃ after stirring and dissolving, begin to slowly drip 5ml concentration of sodium hydroxide aqueous solution that is 50%, stop reaction after 6 hours of reaction, naturally cool to room temperature, suction filter and wash with ethanol, obtain white powdery solid ( sodium chloride and sodium 3,4,5-trimethoxymandelate), recrystallized with water to obtain white crystals, namely sodium 3,4,5-trimethoxymandelate 3.18g, with a yield of 60.2%.
Embodiment 2
[0041] In a 100ml three-necked flask equipped with a thermometer, reflux condenser and dropping funnel, add 3,4,5-trimethoxybenzaldehyde 3.92g (0.02mol), tetraethylammonium bromide (TEAB) 0.5g and 25ml chloroform , be warming up to 30 ℃ after stirring and dissolving, begin to slowly drip the sodium hydroxide aqueous solution that 2ml concentration is 20%, stop the reaction after 12 hours of reaction, naturally cool to room temperature, suction filter and wash with ethanol, obtain white powdery solid ( sodium chloride and sodium 3,4,5-trimethoxymandelate), recrystallized from water-ethanol to obtain white crystals, namely sodium 3,4,5-trimethoxymandelate 2.98g, with a yield of 56.4%.
Embodiment 3
[0043] In a 100ml three-necked flask equipped with a thermometer, reflux condenser and dropping funnel, add 3,4,5-trimethoxybenzaldehyde 3.92g (0.02mol), hexadecyltriethylammonium bromide (CTMAB) 0.5 g and 25ml of chloroform, stir and dissolve and then heat up to 80°C, start to slowly dropwise add 8ml of aqueous sodium hydroxide solution with a concentration of 50%, stop the reaction after 2 hours, cool to room temperature naturally, filter with suction and wash with ethanol to obtain a white Powdered solids (sodium chloride and sodium 3,4,5-trimethoxymandelate) were acidified by adding 1:1 hydrochloric acid to pH=2~3, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, concentrated, and then extracted with anhydrous magnesium sulfate. Recrystallization from ethyl acetate-petroleum ether gave a yellowish solid, which was 2.91 g of 3,4,5-trimethoxymandelic acid, and the yield was 60.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com