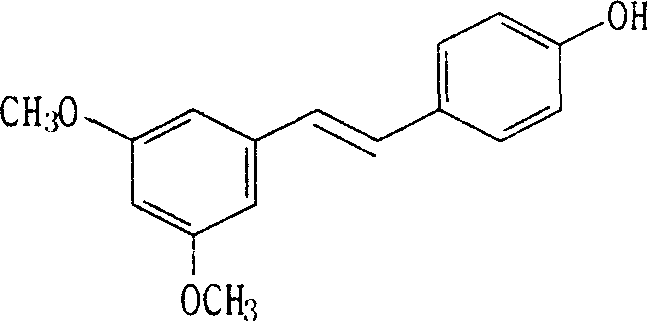
(E)-3,5-dimethox-4'-hydroxy diphenyl ethylene synthesis method
A technology of hydroxystilbene and dimethoxyphenyl, which is applied in the -3 field, can solve problems such as limited sources, and achieve the effects of less waste residue, mild reaction conditions, and favorable industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
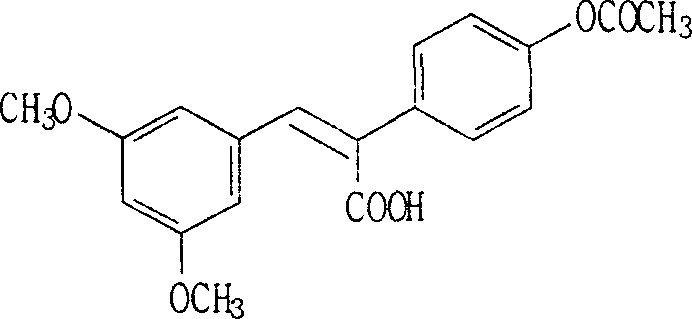
[0034] (1) Synthesis of (Z)-2-(4-acetoxyphenyl)-3-(3,5-dimethoxyphenyl)acrylic acid: 3,5-dimethoxybenzaldehyde (16.6g , 0.1mol), a mixed solution of p-hydroxyphenylacetic acid (15.2g, 0.1mol), acetic anhydride (30g) and triethylamine (25g) was reacted at 120°C for 8 hours. Obtained a light yellow solid, namely (Z)-2-(4-acetoxyphenyl)-3-(3,5-dimethoxyphenyl)acrylic acid 33.86g, yield 99%;
[0035] MS m / z: 342 (M + ), 300, 255, 240, 225, 134.
[0036] 1 H NMR (400Hz, CDCl 3 )2.280 (s, 3H, 4'-COCH 3); 3.514 (s, 6H, 3, 5-CH 3 ); 6.237-6.242 (d, 2H, J = 2.0Hz, 2, 6-Ar-H); 6.335-6.346 (t, 1H, J = 2.0Hz, 4-Ar-H); 7.079-7.106 (d, 2H, J = 6.8Hz, 3', 5'-Ar-H); 7.255-7.282 (d, 2H, J = 6.8Hz, 2', 6'-Ar-H); 7.864 (s, 1H, =CH ).
[0037] IR (KBr, cm -1 ): 3500-2500, 2941, 2841, 1672, 1591, 1510, 1460, 1206.
[0038] (2) Synthesis of (E)-3,5-dimethoxy-4'-acetoxystilbene: (Z)-2-(4-acetoxyphenyl)-3-(3,5 -Dimethoxyphenyl) acrylic acid (3.42g, 0.01mol), copper powder (3.0g) and quinol...
Embodiment 2
[0047] (1) Synthesis of (Z)-2-(4-hydroxyphenyl)-3-(3,5-dimethoxyphenyl)acrylic acid: 3,5-dimethoxybenzaldehyde (16.6g, 0.1 mol), p-hydroxyphenylacetic acid (15.2g, 0.1mol), acetic anhydride (30g) and triethylamine (25g) were reacted at 120°C for 8 hours, and poured into ice water after the reaction was completed, and the solid obtained by suction filtration Pour directly into 150ml of 5% sodium hydroxide solution by mass, stir at 25°C for 3 hours, add 37% hydrochloric acid to acidify to weak acidity (PH5-6), and obtain 29.52g of light yellow solid It is (Z)-2-(4-hydroxyphenyl)-3-(3,5-dimethoxyphenyl)acrylic acid, and the yield is 98.4%.
[0048] MS m / z: 300(M + ), 282, 271, 255, 240, 225, 210, 181, 139, 134.
[0049] IR (KBr, cm -1 ): 3431, 3008, 2949, 1705, 1591, 1512, 1462, 1211, 1159, 833.
[0050] (2) Synthesis of pterostylbene: (Z)-2-(4-hydroxyphenyl)-3-(3,5-dimethoxyphenyl)acrylic acid (3.00g, 0.01mol), copper powder (3.0g ) and quinoline (30.0g) were reacted at 200...
Embodiment 3
[0052] (1) Synthesis of (Z)-2-(4-acetoxyphenyl)-3-(3,5-dimethoxyphenyl)acrylic acid: 3,5-dimethoxybenzaldehyde (16.6g , 0.1mol), a mixed solution of p-hydroxyphenylacetic acid (15.2g, 0.1mol), acetic anhydride (30g) and triethylamine (25g) was reacted at 80°C for 10 hours, and after the reaction was completed, it was directly poured into ice water to obtain a large amount of Light yellow solid 27.65g, yield 80.8%.
[0053] (2) Synthesis of (E)-3,5-dimethoxy-4'-acetoxystilbene: (Z)-2-(4-acetoxyphenyl)-3-(3,5 -dimethoxyphenyl) acrylic acid (3.42g, 0.01mol), copper powder (3.0g) and quinoline (30.0g) were reacted at 150°C for 5 hours, cooled to room temperature, added 50ml ethyl acetate, mass percent Wash with 17% hydrochloric acid until the water layer is nearly colorless, wash with 5% sodium hydroxide solution until neutral, dry the organic layer over anhydrous magnesium sulfate, and concentrate to obtain a solid (E)-3,5 -Dimethoxy-4'-acetoxystilbene, recrystallized to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com