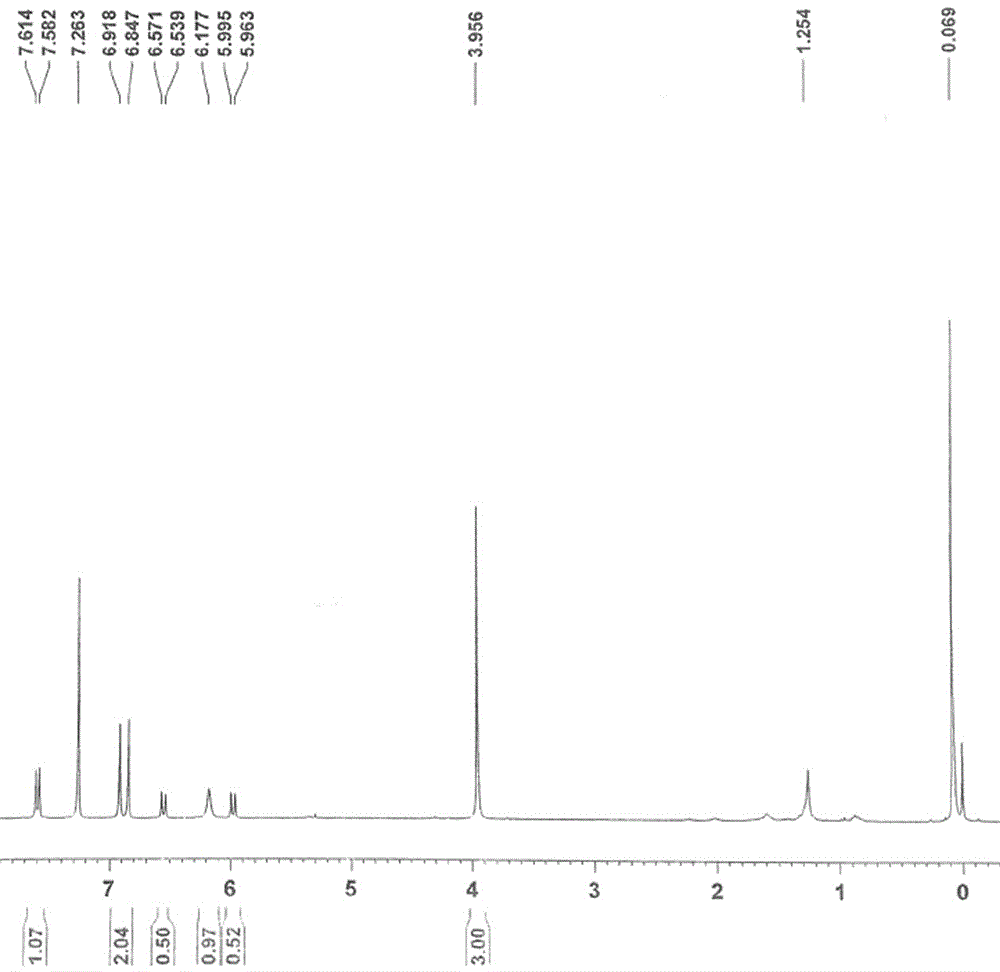
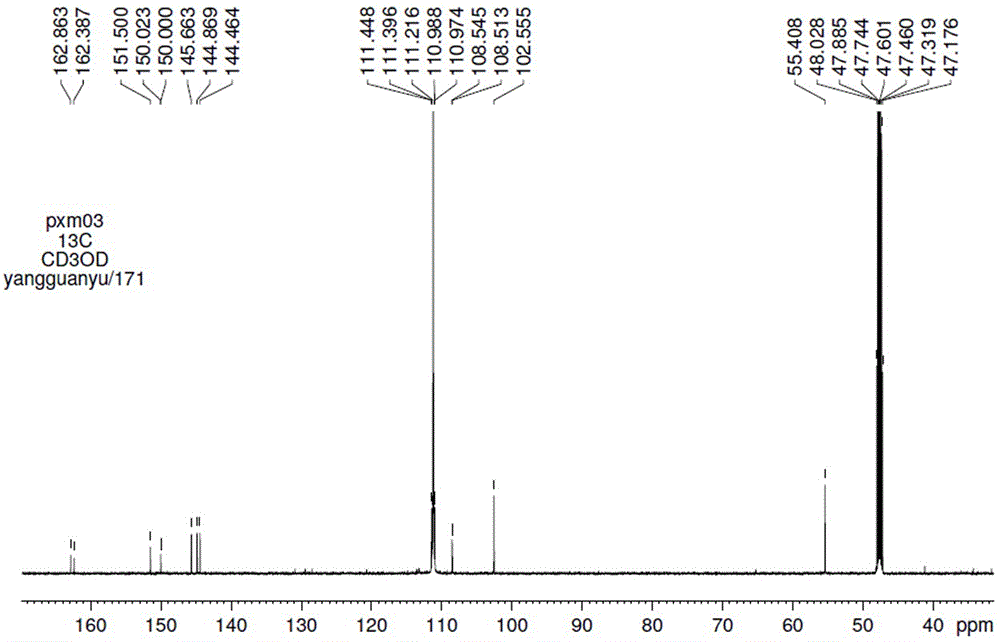
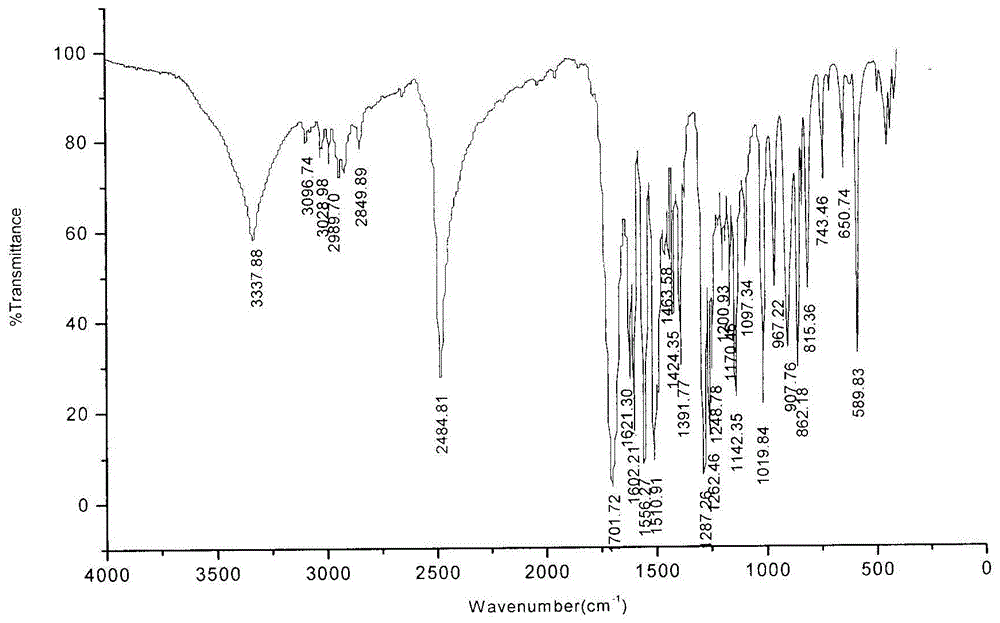
Scopoletin labeled by stable isotope and synthetic method for scopoletin
A stable isotope and isotope labeling technology, which is applied in the direction of isotope introduction of heterocyclic compounds, organic chemistry methods, organic chemistry, etc., can solve the problems of isotope-labeled scopoletin research and no reports, etc., and achieve high product yield and easy operation Effects that are easy to implement and synthesize with simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Isotope-labeled acetic acid reacts with thionyl chloride at a molar ratio of 1:1.5 to generate isotopically-labeled acetyl chloride. The reaction temperature is 60°C for 3 hours, and the fraction at 45°C is distilled.
[0035] (2) In the organic solvent dichloromethane, 2,4-dihydroxy-5-methoxybenzaldehyde reacts with isotope-labeled acetyl chloride (commercial products can also be used directly) in a molar ratio of 3:1 to produce 2- Aldehyde-4-methoxy-5-hydroxyphenylacetate, the concentration of the reaction system (equivalent to the concentration of 2,4-dihydroxy-5-methoxybenzaldehyde in the system) is 0.25M, and the reaction temperature is 20 o C; the reaction time was 17 h; after the reaction, 0.1M hydrochloric acid was added to the reaction mixture to quench the reaction, extracted with dichloromethane, and the obtained crude product was separated and purified by column chromatography to obtain the isotope labeled 2-aldehyde phenyl-4-methoxy-5-hydroxyacetate. ...
Embodiment 2
[0038] (1) The isotope-labeled acetic acid reacts with thionyl chloride at a molar ratio of 1:2.1 to generate isotopically-labeled acetyl chloride. The reaction temperature is 80°C for 2 hours, and the fraction at 43°C is distilled.
[0039] (2) In the organic solvent tetrahydrofuran, 2,4-dihydroxy-5-methoxybenzaldehyde reacts with isotope-labeled acetyl chloride in a molar ratio of 3:2 to generate 2-aldehyde-4-methoxy-5- Phenyl hydroxyacetate, the concentration of the reaction system (equivalent to the concentration of 2,4-dihydroxy-5-methoxybenzaldehyde in the system) is 0.1M, and the ratio is the reaction temperature of 50 oC; the reaction time was 12 h; after the reaction was completed, 1M sulfuric acid was added to the reaction mixture to quench the reaction, extracted with tetrahydrofuran, and the obtained crude product was separated and purified by column chromatography to obtain the isotope labeled 2-aldehyde-4 -Methoxy-5-hydroxyphenylacetate.
[0040] (3) In the org...
Embodiment 3
[0042] (1) Isotope-labeled acetic acid reacts with thionyl chloride at a molar ratio of 1:1.1 to generate isotopically-labeled acetyl chloride. The reaction temperature is 70°C for 3 hours, and the fraction at 51°C is distilled.
[0043] (2) In the organic solvent chloroform, 2,4-dihydroxy-5-methoxybenzaldehyde reacts with isotope-labeled acetyl chloride in a molar ratio of 3:1.4 to generate 2-aldehyde-4-methoxy-5- Phenyl hydroxyacetate, the concentration of the reaction system (equivalent to the concentration of 2,4-dihydroxy-5-methoxybenzaldehyde in the system) is 3M, and the reaction temperature is 35 o C; the reaction time is 20 hours; after the reaction is completed, 2M nitric acid is added to the reaction mixture to quench the reaction, extracted with chloroform, and the obtained crude product is separated and purified by column chromatography to obtain the isotope label 2-aldehyde-4 -Methoxy-5-hydroxyphenylacetate.
[0044] (3) In the organic solvent chloroform, the is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com