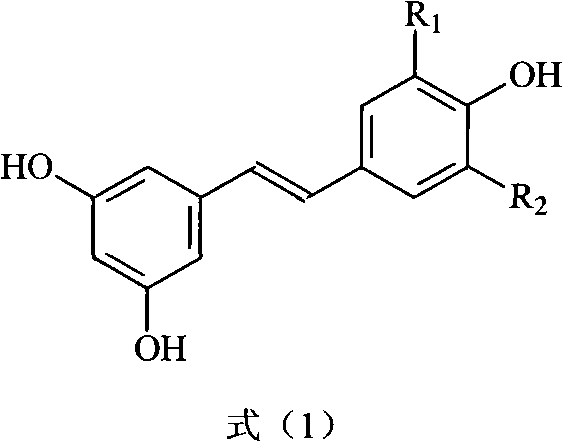
Method for preparing trans polyhydroxystilbene compounds
A technology of polyhydroxystilbene and dihydroxyphenylacetic acid, which is applied in the field of preparation of trans polyhydroxystilbene compounds, can solve the problems of difficult separation, cumbersome steps, and few synthesis studies, and achieve good atom economy, Simple post-processing, less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
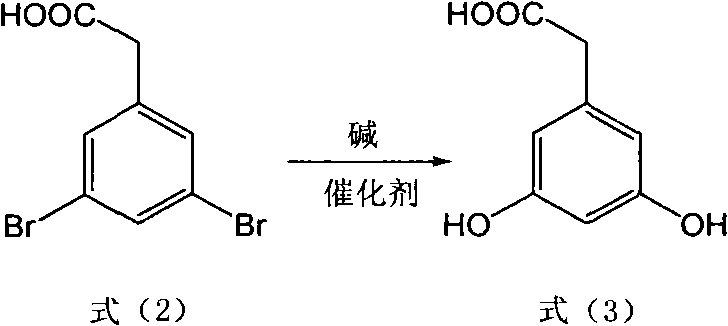
[0039] (1) Take 29.4g (0.1mol) of 3,5-dibromophenylacetic acid, 40g (1mol) of NaOH, CuSO 4 .5H 2 O25.0g (0.1mol), add H 2 080ml in a 250ml stainless steel reactor equipped with a thermometer and a reflux condenser, under nitrogen protection, stirred and heated at 110°C for 72 hours, cooled the reaction solution with suction and filtered, acidified the filtrate with hydrochloric acid to adjust the pH to 4-5, extracted with ethyl acetate, and separated the organic layer, dried, and concentrated under reduced pressure to obtain crude product 3,5-dihydroxyphenylacetic acid 15.1g, yield 90%; MS m / z: 168 (M + ).
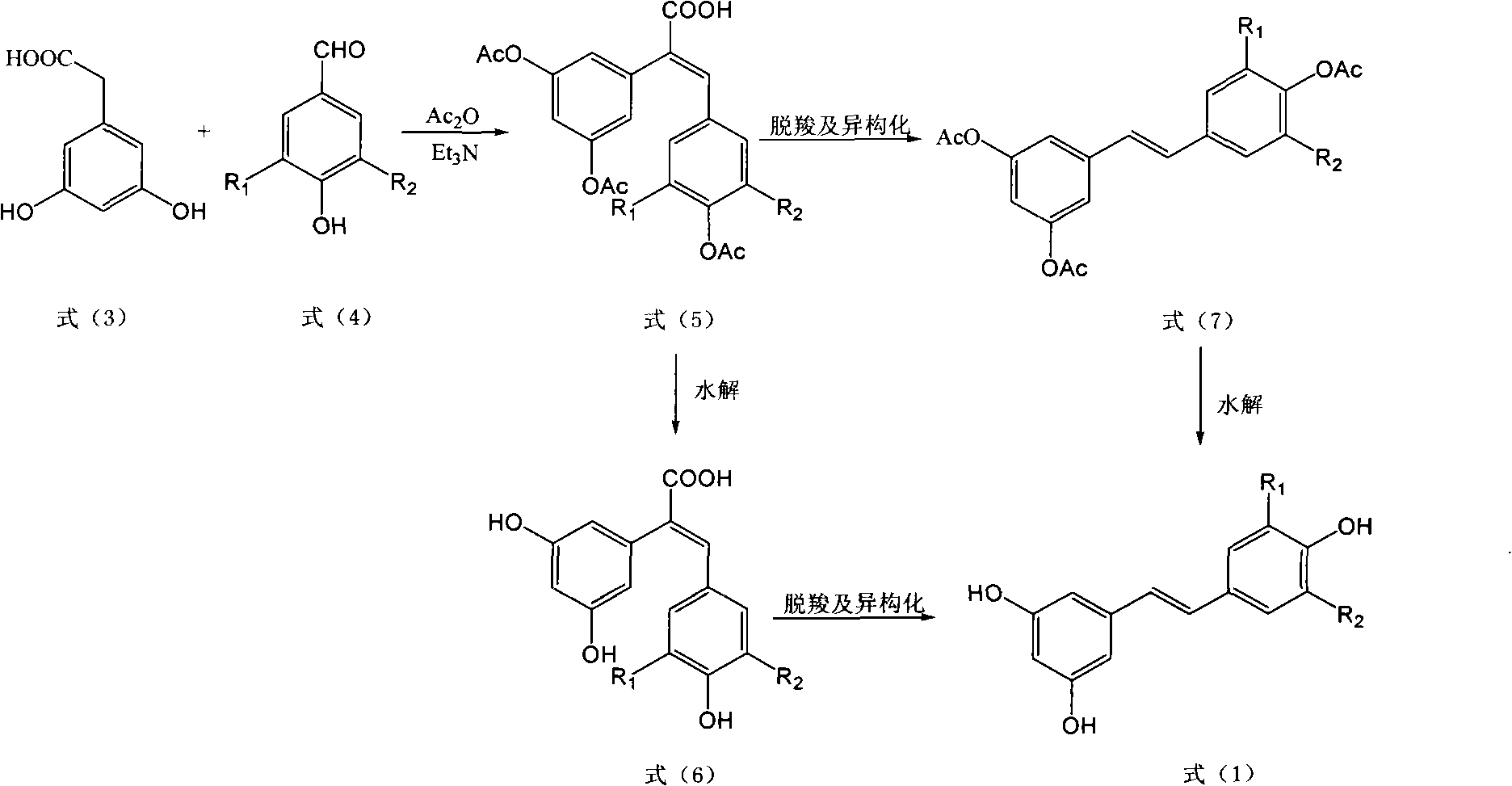
[0040] (2) Weigh 1.68g (10mmol) of 3,5-dihydroxyphenylacetic acid, 1.46g (12mmol) of 4-hydroxybenzaldehyde and add in the there-necked flask equipped with a thermometer and a reflux condenser, then add 3.03g of triethylamine ( 30mmol), 6.12g (60mmol) of acetic anhydride, refluxed at 120°C for 6 hours, after the reaction was complete, cooled the reaction solution and pou...
Embodiment 2
[0045] (1) Take 29.4g (0.1mol) of 3,5-dibromophenylacetic acid, 48g (1.2mol) of NaOH, CuSO 4 16.0g (0.1mol), add H 2 O100ml is placed in a 250ml stainless steel reactor equipped with a thermometer and a reflux condenser. Under nitrogen protection, stir and heat at 110°C for 70 hours. layer, dried, and concentrated under reduced pressure to obtain 15.6 g of crude product 3,5-dihydroxyphenylacetic acid, with a yield of 93%.
[0046] (2) Weigh 1.68g (10mmol) of 3,5-dihydroxyphenylacetic acid, 1.52g (10mmol) of 3-methoxy-4-hydroxybenzaldehyde and add it into a three-necked flask equipped with a thermometer and a reflux condenser, and then add 2.53g (25mmol) of triethylamine, 3.06g (30mmol) of acetic anhydride, refluxed at 110°C for 8 hours. After the reaction was complete, the reaction solution was cooled and poured into water to stir, and a large amount of solids were precipitated. The cake was dried and then recrystallized to obtain 3.42 g of E-2-(3,5-diacetoxyphenyl)-3-(3'-m...
Embodiment 3
[0051] (1) Take 29.4g (0.1mol) of 3,5-dibromophenylacetic acid, KOH56g (1mol), CuSO 4 .5H 2 O25.0g (0.1mol), add H 2 O150ml is placed in a 250ml stainless steel reactor equipped with a thermometer and a reflux condenser. Under nitrogen protection, stir and heat at 120°C for 60 hours. layer, dried, and concentrated under reduced pressure to obtain crude product 3,5-dihydroxyphenylacetic acid 15.8g, yield 94%;
[0052] (2) Weigh 1.68g (10mmol) of 3,5-dihydroxyphenylacetic acid and 1.38g (10mmol) of 3,4-dihydroxybenzaldehyde into a three-necked flask equipped with a thermometer and a reflux condenser, and then add triethylamine 4.04g (40mmol), 6.12g (60mmol) of acetic anhydride, reflux at 115°C for 7 hours. After the reaction is complete, cool the reaction solution and pour it into water to stir. A large amount of solids are precipitated. After standing for a while, suction filter and dry the filter cake After recrystallization, 3.60 g of E-2-(3,5-diacetoxyphenyl)-3-(3',4'-dia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com