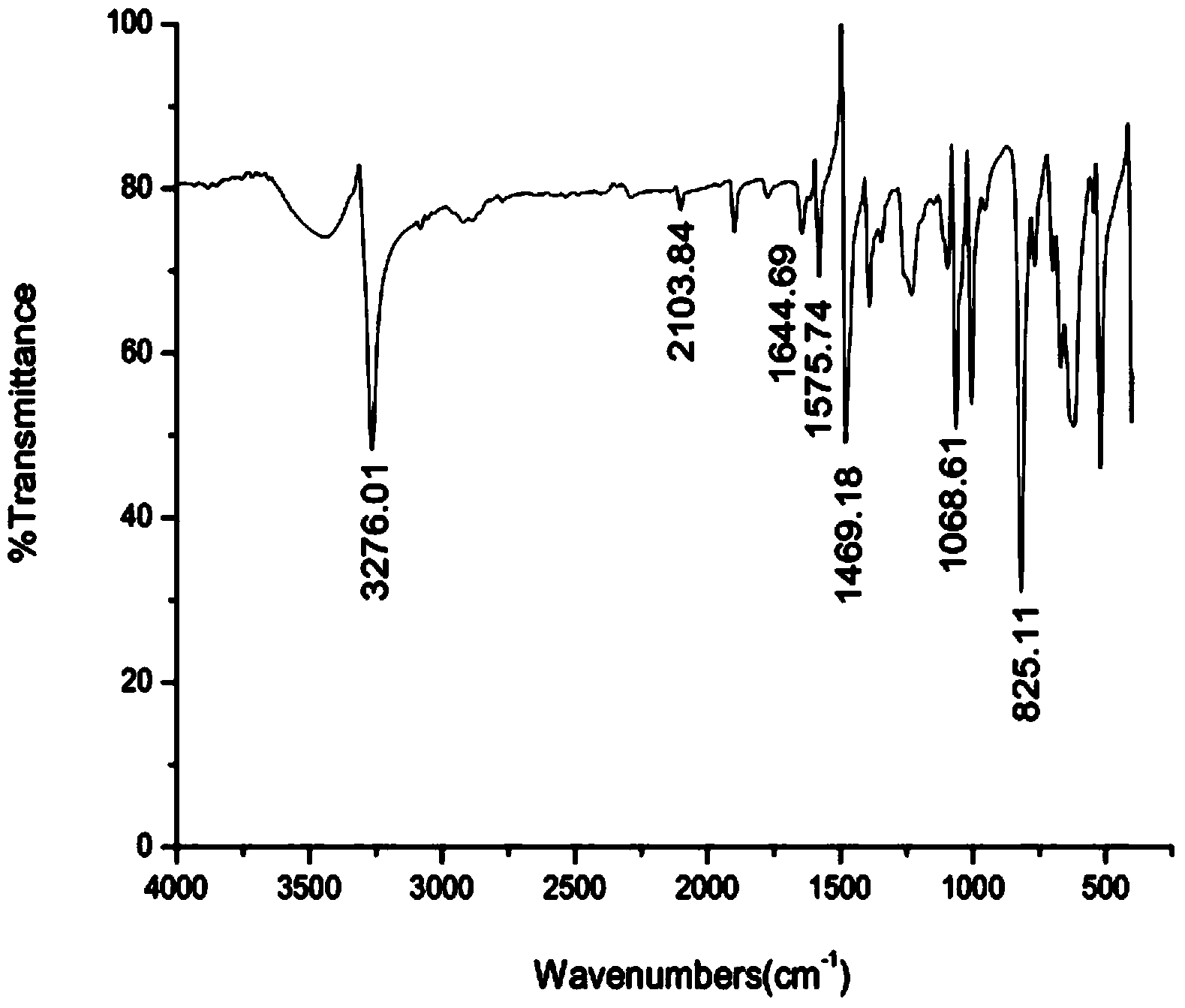
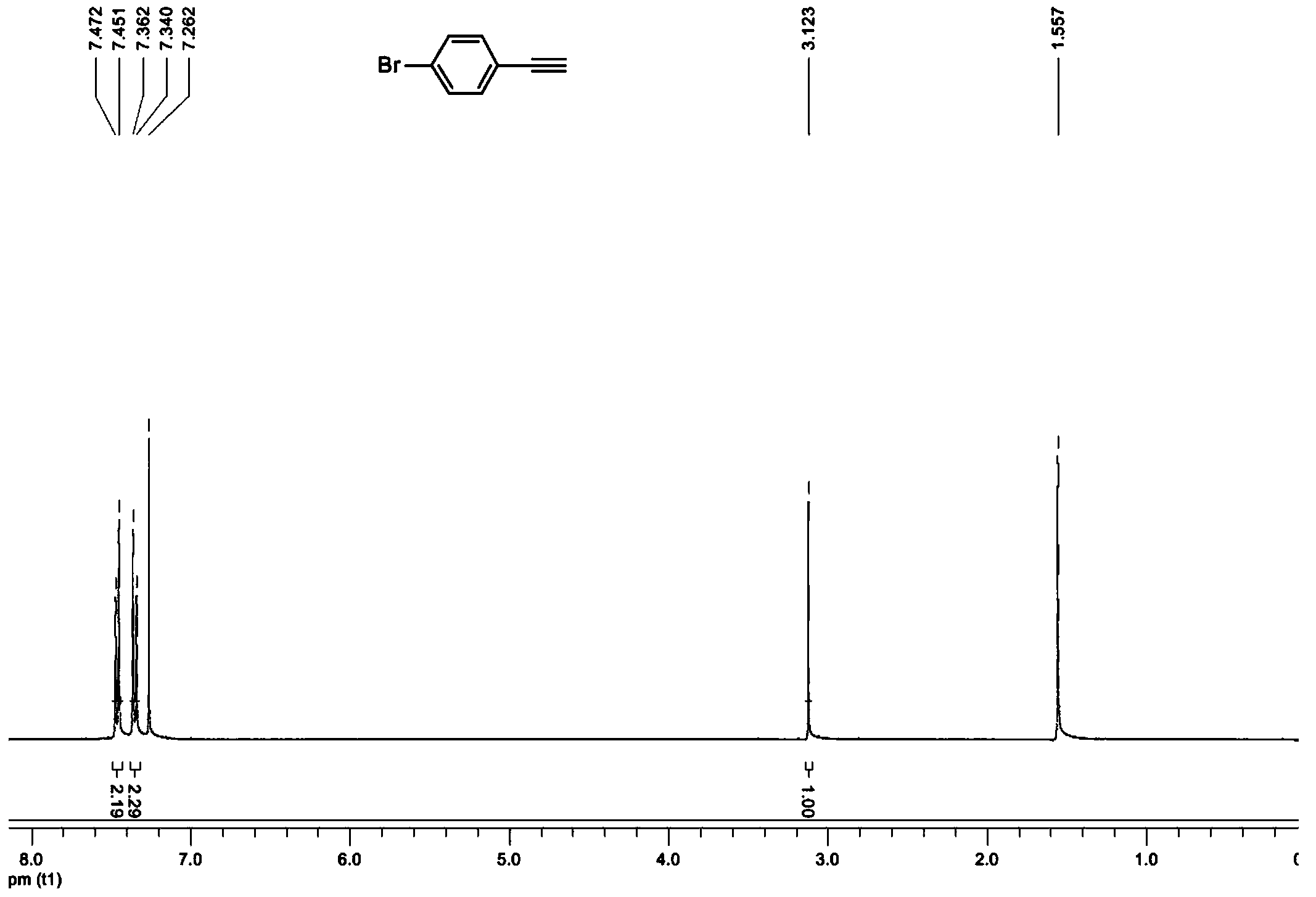
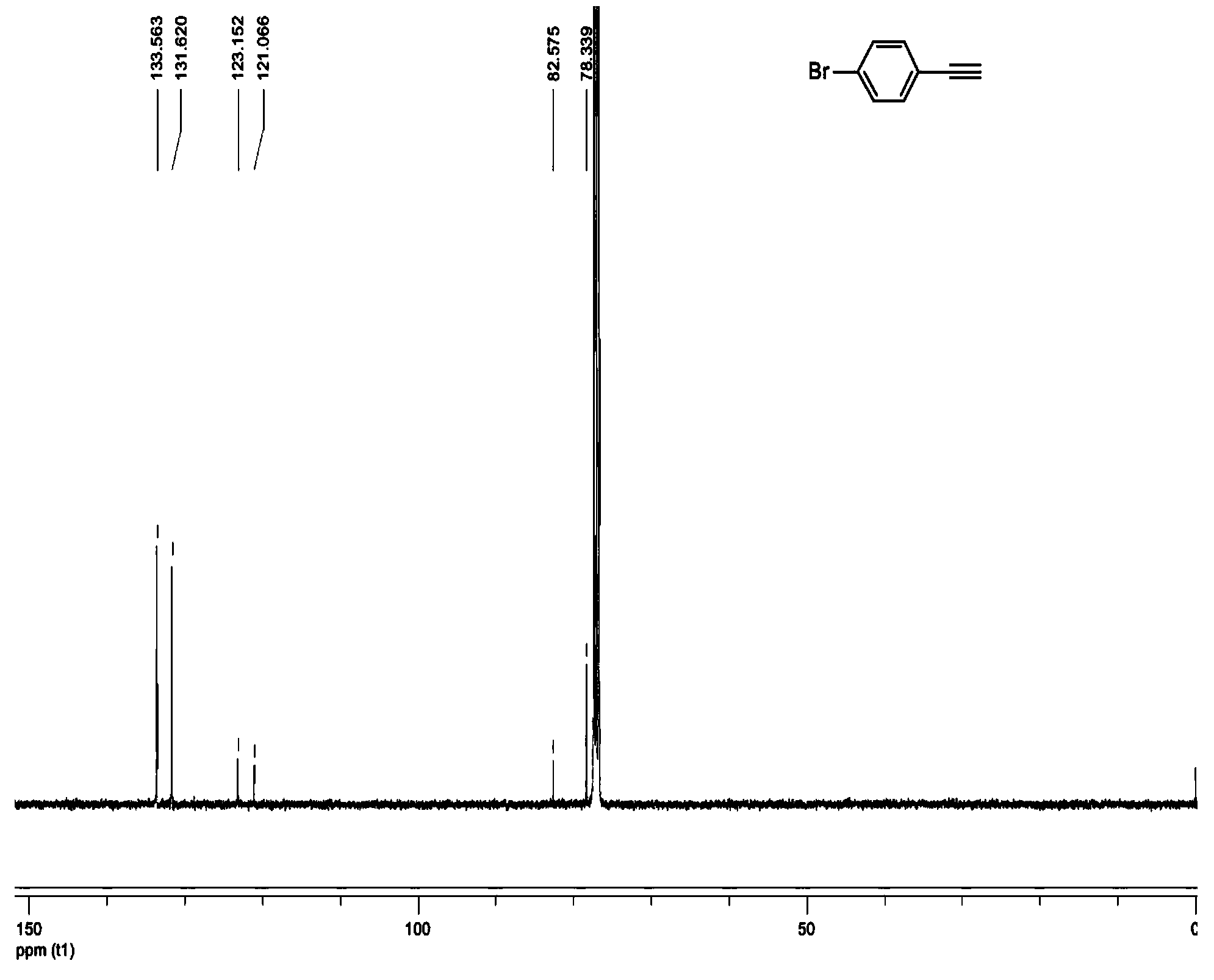
New synthetic method of 4-halogenated phenylacetylene
A technology of halogenated benzene and a new method, which is applied in the field of synthesis of halogenated phenylacetylenes, can solve the problems of low yield of target products, difficult purification of products, and great environmental hazards, and achieve high product purity, easy control, and high yield reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062]
[0063] In the first step, dissolve 4-bromobenzaldehyde in 4 equivalents of acetic anhydride, add 1.5 equivalents of anhydrous potassium carbonate, control the temperature at 130-140 degrees, monitor by TLC until the conversion of the substrate is complete, and isolate product 1;
[0064] In the second step, the product 1 was dissolved in an appropriate amount of glacial acetic acid, and 1.5 equivalents of liquid bromine was added dropwise thereto under the condition of 60 degrees, and the product 2 was isolated by TLC monitoring until the conversion of the substrate was complete;
[0065] In the third step, the product 2 was dissolved in an appropriate amount of acetone, and 3 equivalents of sodium bicarbonate was added thereto under the condition of 55-60 degrees, and the product 3 was isolated by TLC monitoring until the conversion of the substrate was complete;
[0066] In the fourth step, the product 3 was dissolved in an appropriate amount of ethanol, and 3 equ...
Embodiment 2
[0068]
[0069] In the first step, dissolve 4-bromobenzaldehyde in 4 equivalents of acetic anhydride, add 1.5 equivalents of anhydrous sodium carbonate, control the temperature at 130-140 degrees, monitor by TLC until the conversion of the substrate is complete, and isolate product 1;
[0070] In the second step, the product 1 was dissolved in an appropriate amount of glacial acetic acid, and 1.5 equivalents of liquid bromine was added dropwise thereto under the condition of 60 degrees, and the product 2 was isolated by TLC monitoring until the conversion of the substrate was complete;
[0071] In the third step, the product 2 was dissolved in an appropriate amount of acetone, and 3 equivalents of sodium bicarbonate was added thereto under the condition of 55-60 degrees, and the product 3 was isolated by TLC monitoring until the conversion of the substrate was complete;
[0072] In the fourth step, the product 3 was dissolved in an appropriate amount of ethanol, and 3 equiva...
Embodiment 3
[0074]
[0075] In the first step, dissolve 4-bromobenzaldehyde in 4 equivalents of acetic anhydride, add 1.5 equivalents of anhydrous sodium bicarbonate, control the temperature at 130-140 degrees, monitor by TLC until the conversion of the substrate is complete, and isolate product 1;
[0076] In the second step, the product 1 was dissolved in an appropriate amount of glacial acetic acid, and 1.5 equivalents of liquid bromine was added dropwise thereto under the condition of 60 degrees, and the product 2 was isolated by TLC monitoring until the conversion of the substrate was complete;
[0077] In the third step, the product 2 was dissolved in an appropriate amount of acetone, and 3 equivalents of sodium bicarbonate was added thereto under the condition of 55-60 degrees, and the product 3 was isolated by TLC monitoring until the conversion of the substrate was complete;
[0078] In the fourth step, the product 3 was dissolved in an appropriate amount of ethanol, and 3 equi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com