Method for preparing caffeic acid derivative
A technology of derivatives and caffeic acid, applied in the field of preparation of caffeic acid derivatives, can solve the problem of no synthesis method and the like, and achieve the effects of low production cost, high product yield and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
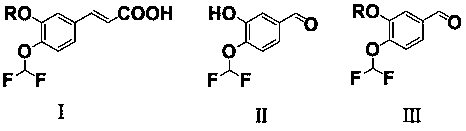
[0018] The preparation method of the caffeic acid derivative of the present invention is to use the compound shown in the formula II as a raw material to undergo a substitution reaction with a halogenated alkane to obtain the compound shown in the formula III, and then carry out the Perkin reaction with acetic anhydride to obtain the compound shown in the formula I The compounds shown are the target caffeic acid derivatives, wherein the structures of the compounds shown in formula I, formula II and formula III are as follows:
[0019]
[0020] In the formula: R represents methyl or cyclopropyl methylene.
[0021] The substitution reaction is to dissolve the raw material and 3-hydroxy-4-difluoromethoxybenzaldehyde in an organic solvent, react at 50-85°C for 2-6 hours under the action of an alkali catalyst, filter the reaction solution, and filter the residue with di Wash and concentrate with methyl chloride, add water to the concentrated solution, extract with dichloromethan...
Embodiment 1
[0032] Example 1 Preparation of 3-cyclopropylideneoxy-4-difluoromethoxycinnamic acid
[0033] a. Preparation of intermediate 3-cyclopropylideneoxy-4-difluoromethoxybenzaldehyde
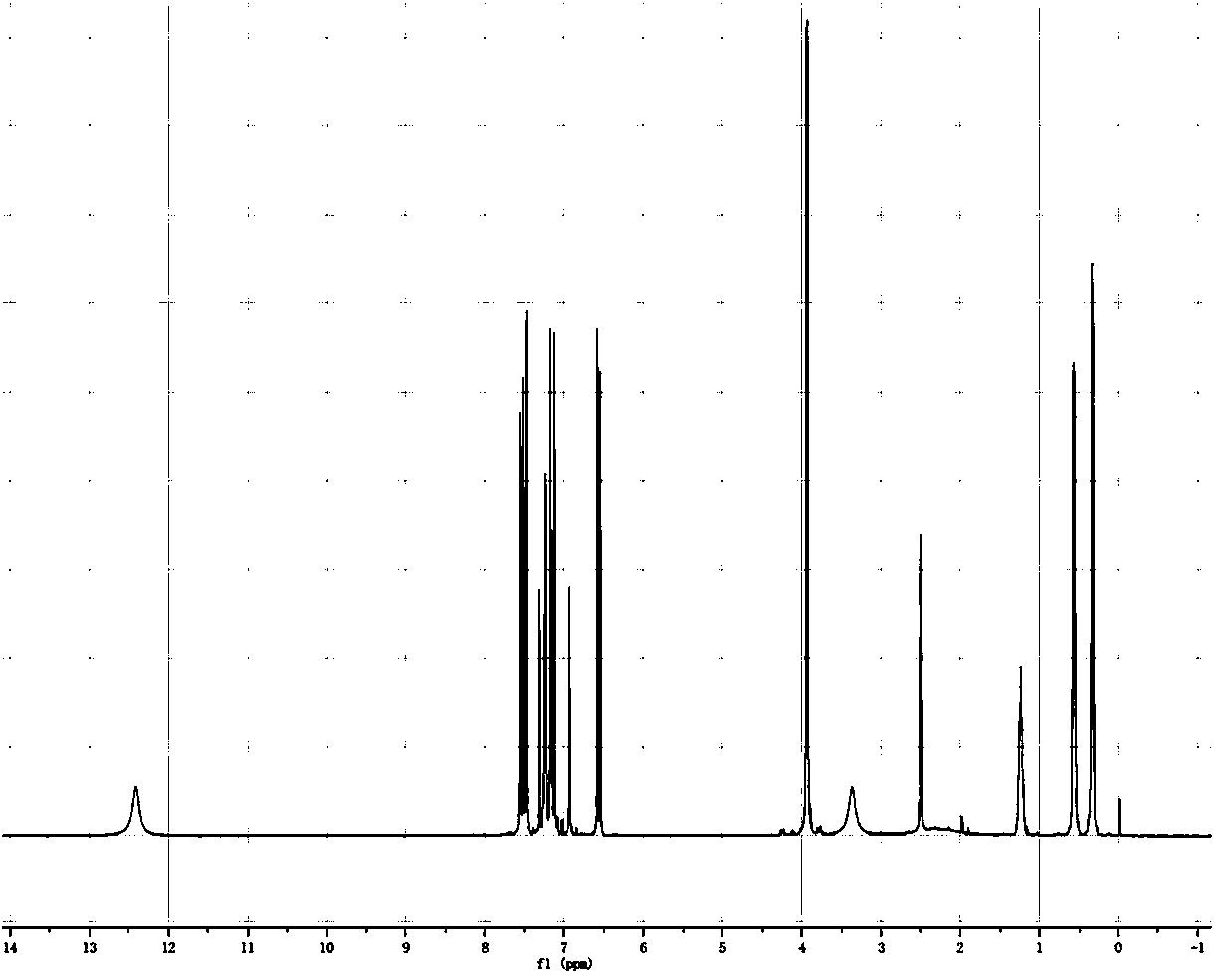
[0034] In a reaction flask, add 3-hydroxy-4-difluoromethoxybenzaldehyde (21g, 0.11mol), cyclopropylmethyl bromide (18.1g, 0.13mol), K 2 CO 3 (18.5g), dissolved in 199.5gDMF, reacted for 2h under stirring at 85°C. After TLC monitoring until the reaction is complete, filter the reaction solution, and use CH 2 Cl 2 Wash and concentrate, add CH to the concentrate 2 Cl 2 、H 2 O liquid extraction, take the organic phase with anhydrous MgSO 4 After drying and concentrating, 31 g of light yellow 3-cyclopropylideneoxy-4-(difluoromethoxy)benzaldehyde solution was obtained.
[0035] b. Preparation of 3-cyclopropylideneoxy-4-difluoromethoxycinnamic acid
[0036] In the reaction flask, add 3-cyclopropylideneoxy-4-difluoromethoxybenzaldehyde (2.41g, 0.01mol), acetic anhydride (3.06g, 0.03mol), K 2 CO 3 ...
Embodiment 2
[0039] Example 2 Preparation of 3-methoxy-4-difluoromethoxycinnamic acid
[0040] The preparation of the compound 3-methoxy-4-difluoromethoxycinnamic acid in this example refers to Example 1, and a white solid 3-methoxy-4-difluoromethoxycinnamic acid is prepared, the yield: 79 %.
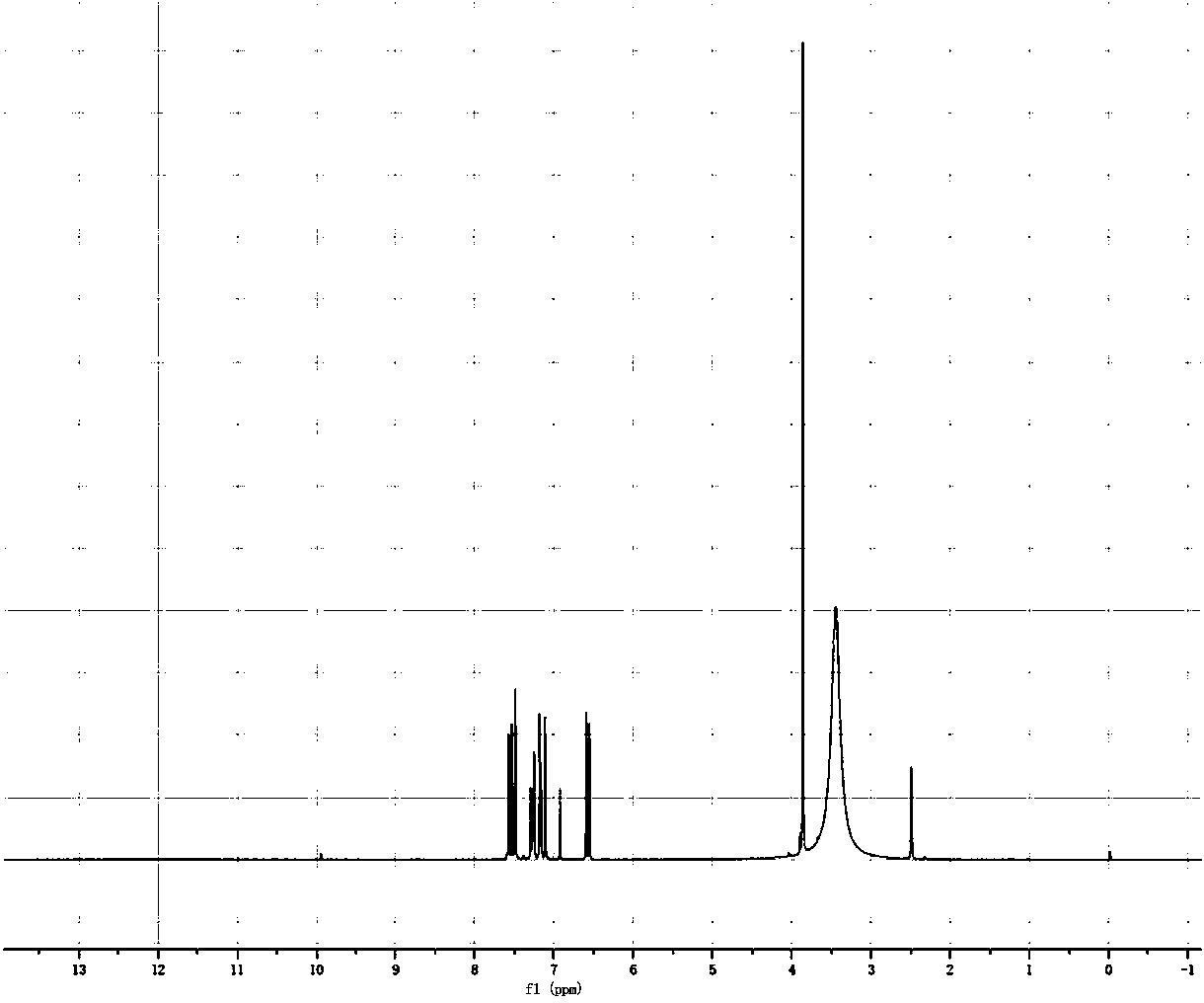
[0041] The compound NMR identification is as follows:
[0042] 1H-NMR (500MHz, CDCl3, δppm): δ11 (s, 1H), δ6.70 (s, 1H), δ6.61 (m, 1H), δ6.75 (m, 1H), δ3.73 (s , 3H), δ7.36 (s, 1H), δ6.41 (d, 1H), δ7.61 (d, 1H). The structural formula is shown in Table 1.
[0043] Table 1 Compound structural formula that embodiment 1~2 makes
[0044] Example number Compound number R structural formula 1 KPC-3000179 2 KPC-3000180
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com