Preparation method of gliclazide
A technology of phenyl and compound, which is applied in the field of drug synthesis and can solve problems such as the difficulty in quality control of Gliclazide API
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
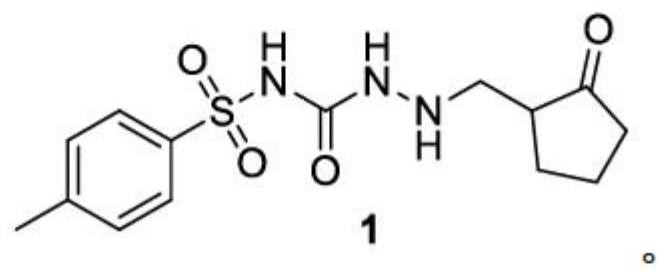
[0087] Embodiment 1: the synthesis of compound 1
[0088]
[0089] N-tosylhydraziecarboxamide (5 g, 0.0218 mol), cyclopentanone (1.83 g, 0.0218 mol), paraformaldehyde (0.5 g) and ethanol (50 mL) were put into a 250 ml four-necked flask. Turn on the magnetic stirring, stir and dissolve at 0-5°C. Concentrated hydrochloric acid (5 mL) was added at 0-5°C. The temperature was raised to reflux for 6 hours, and the conversion of the raw materials was complete. The reaction solution was concentrated to dryness at 35-40°C under reduced pressure, ethyl acetate (30g) and water (30g) were added, the organic phase was separated, ethyl acetate (30g) was added to the aqueous phase, the organic phases were combined, and saturated bicarbonate Wash once with 15g of sodium aqueous solution, separate the organic phase, and concentrate to dryness under reduced pressure at 35-40°C to obtain a yellow viscous substance, add 7g of isopropanol, heat to 75-80°C to dissolve, stop heating, and natura...
Embodiment 2
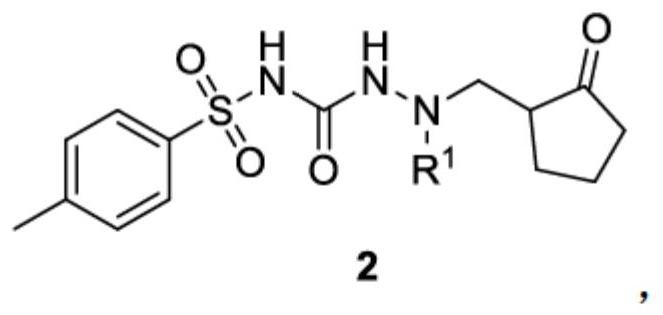
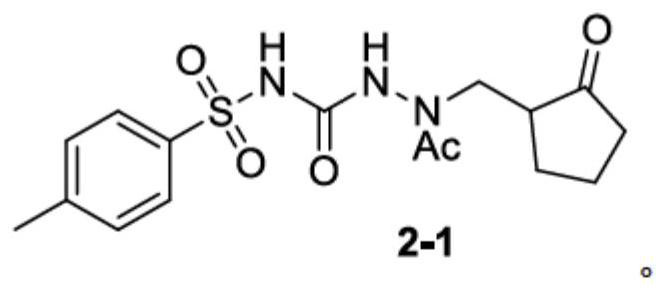
[0090] Embodiment 2: the synthesis of compound 2-1
[0091]
[0092] Compound 1 (3.25 g, 0.01 mol), DCM (30 mL), triethylamine (1.1 g), DMAP (0.12 g) and acetic anhydride (1.12 g) were put into a 100 ml four-neck flask. Turn on magnetic stirring, and react at 20-25°C for 3 hours. After the reaction, add 20 mL of water, separate the organic phase, wash once with 10 mL of saturated sodium bicarbonate solution, separate the organic phase, and concentrate to dryness at 35-40 ° C under reduced pressure to obtain the crude product, then wash with ethyl acetate (3 ml) and n-heptyl The alkane (15ml) was recrystallized to obtain a pale yellow solid (2.64g), namely the target compound 2-1, with a yield of 72%.
Embodiment 3
[0093]Embodiment 3: the synthesis of compound 3-1
[0094]
[0095] Compound 2-1 (4.2 g, 0.011 mol), ethyl chloroacetate (1.34 g), and THF (40 ml) were put into a 100 ml four-neck flask. Turn on magnetic stirring, slowly add t-BuOK (1.85g) at 20-25°C, and react at 20-25°C for 15 hours after the addition is complete. After the reaction, add 20mL saturated ammonium chloride aqueous solution and 20mL ethyl acetate, stir and separate the layers, extract the aqueous phase twice with ethyl acetate (20mL x2), combine the organic phases, and concentrate to dryness under reduced pressure at 35-40°C to obtain Brown oil, the crude product was separated by column chromatography [mobile phase is n-heptane: ethyl acetate = 2: 1 (volume ratio)] to obtain light yellow oil (2.6g), the target product compound 3-1, yield rate 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com