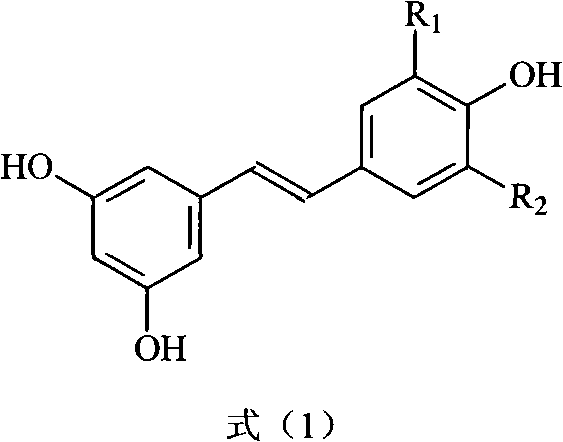
Method for preparing trans polyhydroxystilbene compounds
A technology of polyhydroxystilbene and dihydroxyphenylacetic acid, which is applied in the field of preparation of trans-polyhydroxystilbene compounds, can solve the problems of difficult separation, few synthesis studies, cumbersome steps, etc., and achieve good atom economy, Effects with simple post-processing and short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
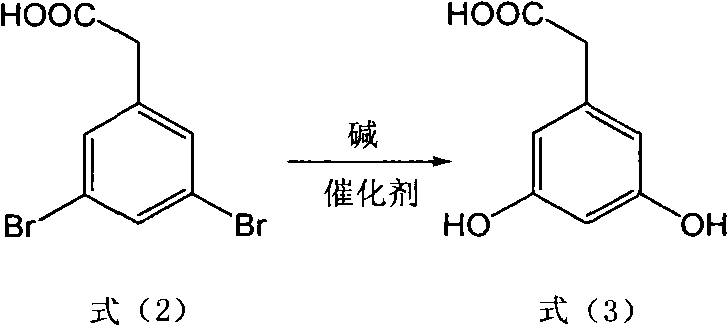
[0039] (1) Take 29.4g (0.1mol) of 3,5-dibromophenylacetic acid, 40g (1mol) of NaOH, CuSO 4 .5H 2 O25.0g (0.1mol), add H 2 080ml in a 250ml stainless steel reactor equipped with a thermometer and a reflux condenser, under nitrogen protection, stirred and heated at 110°C for 72 hours, cooled the reaction solution with suction and filtered, acidified the filtrate with hydrochloric acid to adjust the pH to 4-5, extracted with ethyl acetate, and separated the organic layer, dried, and concentrated under reduced pressure to obtain crude product 3,5-dihydroxyphenylacetic acid 15.1g, yield 90%; MS m / z: 168 (M + ).
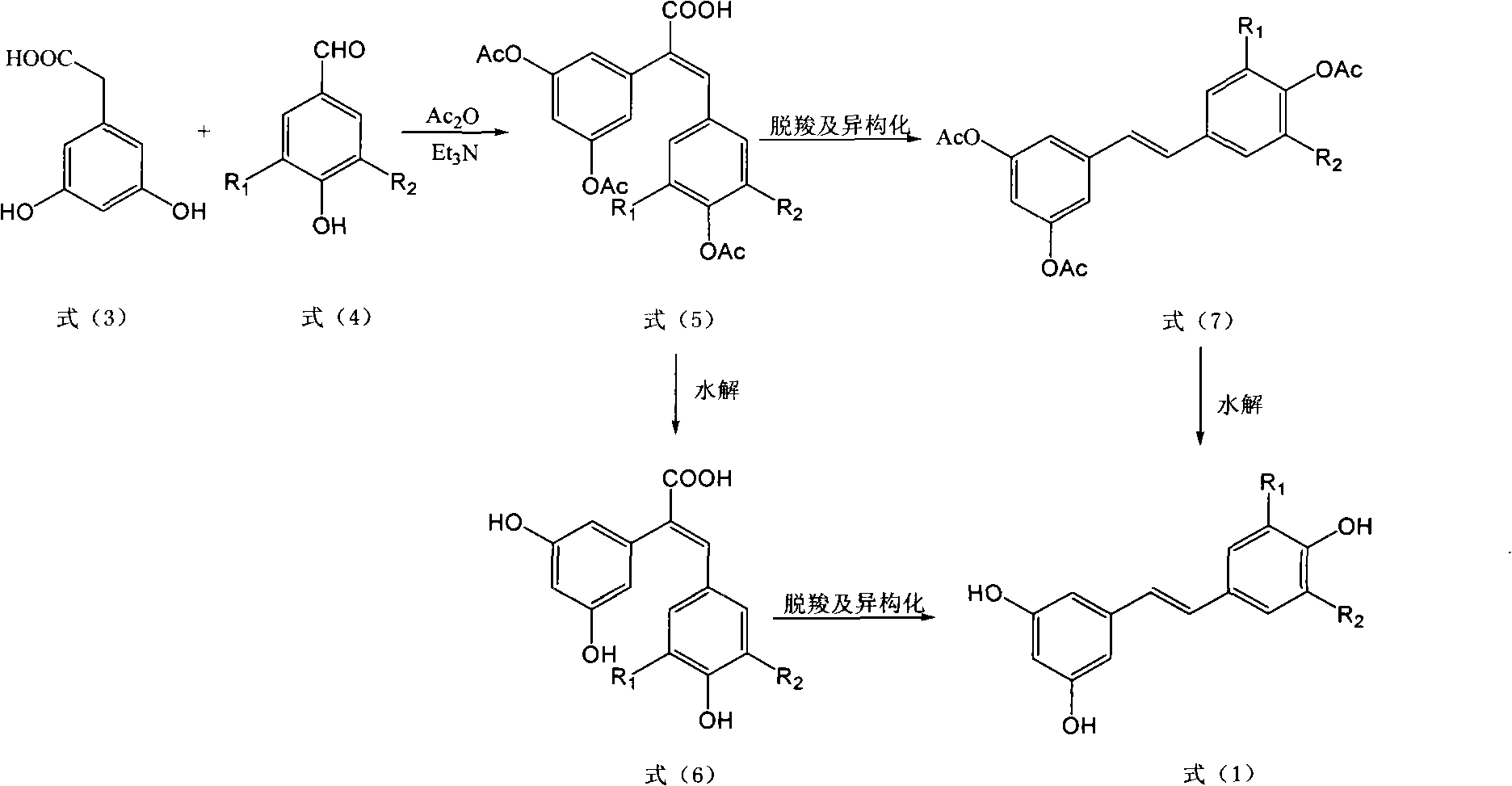
[0040] (2) Weigh 1.68g (10mmol) of 3,5-dihydroxyphenylacetic acid, 1.46g (12mmol) of 4-hydroxybenzaldehyde and add in the there-necked flask equipped with a thermometer and a reflux condenser, then add 3.03g of triethylamine ( 30mmol), 6.12g (60mmol) of acetic anhydride, refluxed at 120°C for 6 hours, after the reaction was complete, cooled the reaction solution and pou...
Embodiment 2
[0045] (1) Take 29.4g (0.1mol) of 3,5-dibromophenylacetic acid, 48g (1.2mol) of NaOH, CuSO 4 16.0g (0.1mol), add H 2 O100ml is placed in a 250ml stainless steel reactor equipped with a thermometer and a reflux condenser. Under nitrogen protection, stir and heat at 110°C for 70 hours. layer, dried, and concentrated under reduced pressure to obtain 15.6 g of crude product 3,5-dihydroxyphenylacetic acid, with a yield of 93%.
[0046] (2) Weigh 1.68g (10mmol) of 3,5-dihydroxyphenylacetic acid, 1.52g (10mmol) of 3-methoxy-4-hydroxybenzaldehyde and add it into a three-necked flask equipped with a thermometer and a reflux condenser, and then add 2.53g (25mmol) of triethylamine, 3.06g (30mmol) of acetic anhydride, refluxed at 110°C for 8 hours. After the reaction was complete, the reaction solution was cooled and poured into water to stir, and a large amount of solids were precipitated. The cake was dried and then recrystallized to obtain 3.42 g of E-2-(3,5-diacetoxyphenyl)-3-(3'-m...
Embodiment 3
[0051] (1) Take 29.4g (0.1mol) of 3,5-dibromophenylacetic acid, KOH56g (1mol), CuSO 4 .5H 2 O25.0g (0.1mol), add H 2 O150ml is placed in a 250ml stainless steel reactor equipped with a thermometer and a reflux condenser. Under nitrogen protection, stir and heat at 120°C for 60 hours. layer, dried, and concentrated under reduced pressure to obtain crude product 3,5-dihydroxyphenylacetic acid 15.8g, yield 94%;
[0052] (2) Weigh 1.68g (10mmol) of 3,5-dihydroxyphenylacetic acid and 1.38g (10mmol) of 3,4-dihydroxybenzaldehyde into a three-necked flask equipped with a thermometer and a reflux condenser, and then add triethylamine 4.04g (40mmol), 6.12g (60mmol) of acetic anhydride, reflux at 115°C for 7 hours. After the reaction is complete, cool the reaction solution and pour it into water to stir. A large amount of solids are precipitated. After standing for a while, suction filter and dry the filter cake After recrystallization, 3.60 g of E-2-(3,5-diacetoxyphenyl)-3-(3',4'-dia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com