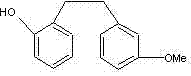
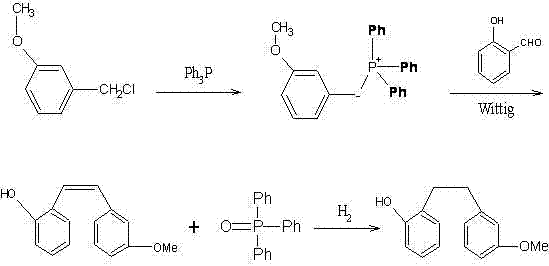
Preparation method of Sarpogrelate intermediate 2-((3-methoxy) phenethyl) phenol
A technology of methoxyphenylacetic acid and sarpogrelate, applied in the field of medicine and chemical industry, can solve problems such as large environmental pollution, and achieve the effects of short process route, low production cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be further described below in conjunction with the examples, but it should not be understood that the scope of the subject of the present invention is limited to the following examples. Without departing from the above-mentioned technical idea of the present invention, various replacements and changes made according to common technical knowledge and conventional means in this field shall be included in the scope of the present invention.
[0032] Implementation column 1:
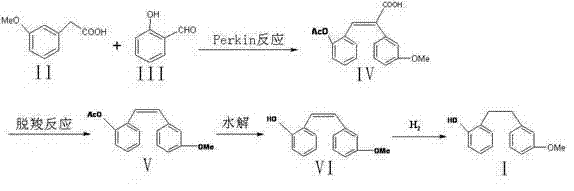
[0033] 1) Preparation of acrylic compound:
[0034] Weigh 16.6 g (0.1 mol) of 3-methoxyphenylacetic acid and 12.2 g (0.1 mol) of salicylaldehyde, add to the reaction flask, dissolve with 40.8 g (0.4 mol) acetic anhydride, then add 25.3 g (0.25 mol) ) triethylamine, heated to 120 ° C for 5 hours, poured into ice water and stirred to precipitate a solid, stood still, and filtered with suction to obtain 2-(3-methoxyphenyl)-3-(2-acetoxyphenyl ) Acrylic acid 26.7g, yield 85....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com