Drugs for prevention and treatment of pulmonary arterial hypertension and their synthesis and application
A technology for drugs and condensation reactions, which can be used in drug combinations, active ingredients of heterocyclic compounds, cardiovascular system diseases, etc. Improve the effect of easy oxidation and promote long-term effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Intermediate 3
[0033]
[0034] Take 8.00g of 3,4-dimethoxyphenylacetic acid (1), 6.00g of p-methoxybenzaldehyde (2), 15mL of acetic anhydride and 6mL of anhydrous triethylamine, stir and heat to 130°C, and reflux for 6h. After standing at room temperature, add 22.00g of potassium carbonate and 90mL of distilled water, stir evenly, and then heat to 130°C for reflux for 0.5h. After standing at room temperature, the pH was adjusted to 4.0 with concentrated hydrochloric acid, the solid was filtered off, and the solid was recrystallized with methanol, with a yield of 46.65%.
[0035] Synthesis of Target Compounds by Acid Chloride Method
[0036]
[0037] Take 6.28g of intermediate 3 in a clean 100mL single-necked bottle, cool to 0°C, add 6mL of SOCl under nitrogen atmosphere 2 , stirred for 15 minutes, removed the nitrogen gas, and heated to reflux at 79°C for 2~3 hours to obtain the acid chloride solution of intermediate 3. After airing at room tem...
Embodiment 2
[0045] Take 8.00g of 3,4-dimethoxyphenylacetic acid (1), 6.00g of p-methoxyphenylacetaldehyde (2), 15mL of acetic anhydride and 6mL of anhydrous pyridine, stir and heat to 130°C, and reflux for 6h. After standing at room temperature, add 22.00g of potassium carbonate and 90mL of distilled water, stir evenly, and then heat to 130°C for reflux for 0.5h. After standing at room temperature, the pH was adjusted to 4.0 with concentrated hydrochloric acid, the solid was filtered out, and the solid was recrystallized with methanol to obtain the following compound with a yield of 46.65%.
[0046]
[0047] Above-mentioned compound further adopts the condensation method similar to embodiment 1 to be able to prepare following compound,
[0048] .
Embodiment 3
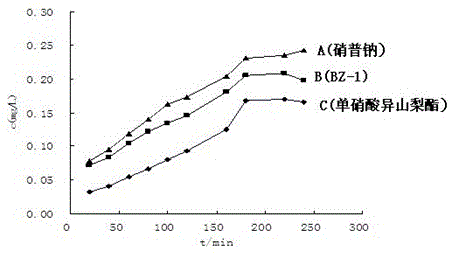
[0049] Example 3: Pharmacological experiments and results of the target compound prepared in Example 1
[0050] 1. Measurement of nitric oxide release in vitro: Nitrate compounds can release NO in the presence of acidic environment and excess mercapto compounds. Nitrite ion NO produced by oxidation of NO 2 - , its concentration is determined by Griess method, which indirectly reflects the nitric oxide release ability of the compound. This method is simple, rapid and reproducible. In this experiment, this method was used to measure the NO release amount of the target compound BZ-1 in vitro to investigate its NO release effect. The control drugs were isosorbide mononitrate and sodium nitroprusside. Using nitrite ion NO 2 - It can undergo diazotization and coupling reactions with Griess reagent to generate a purple-red product. Measure its absorbance value at a wavelength of 540nm to indirectly measure the release of NO. The release environment of NO is the environment of rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com