Isoindoline derivative and preparation method thereof
A technology of isoindolines and indolines, which is applied in the field of isoindoline derivatives and their preparation, can solve the problems of many by-products and poor compatibility, and achieve the effect of increasing complexity and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
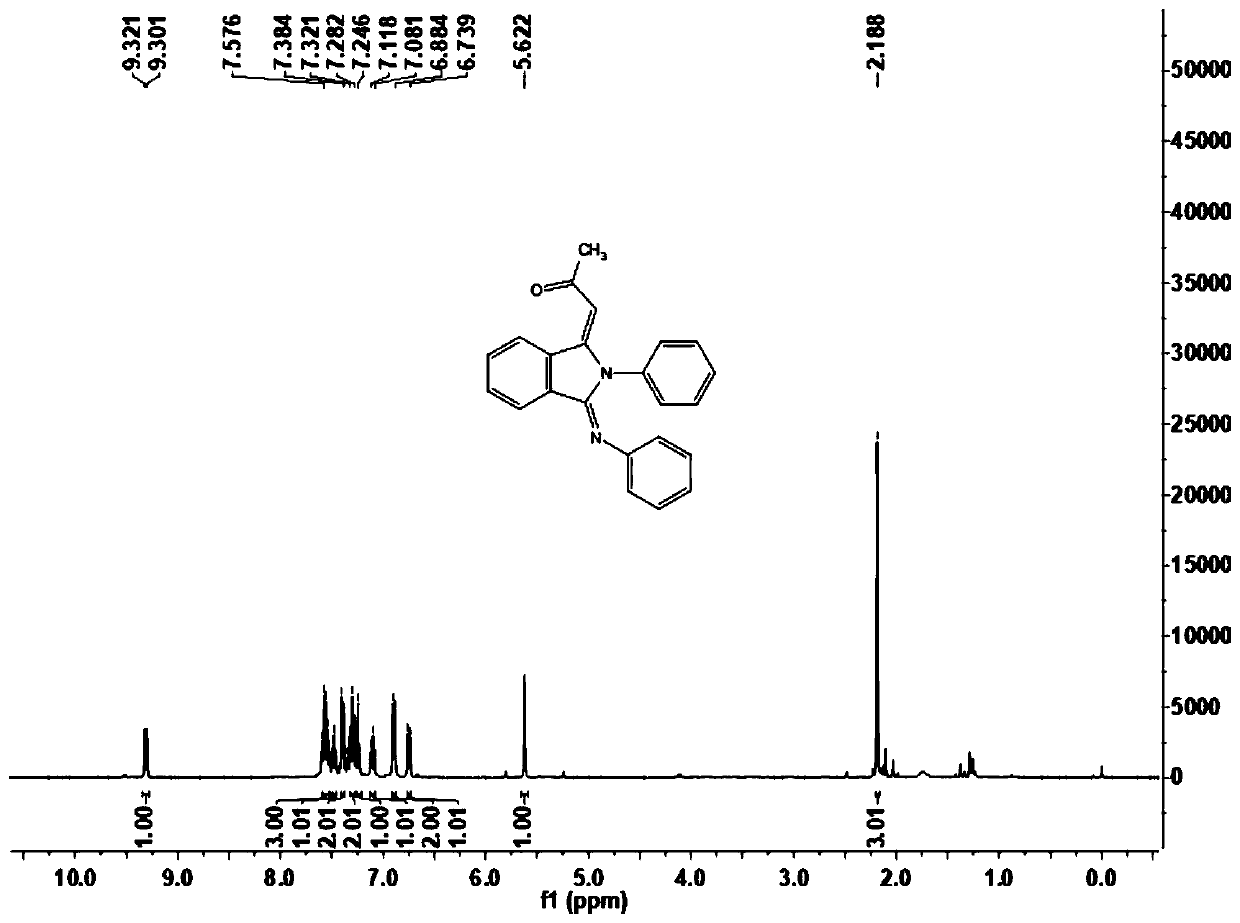
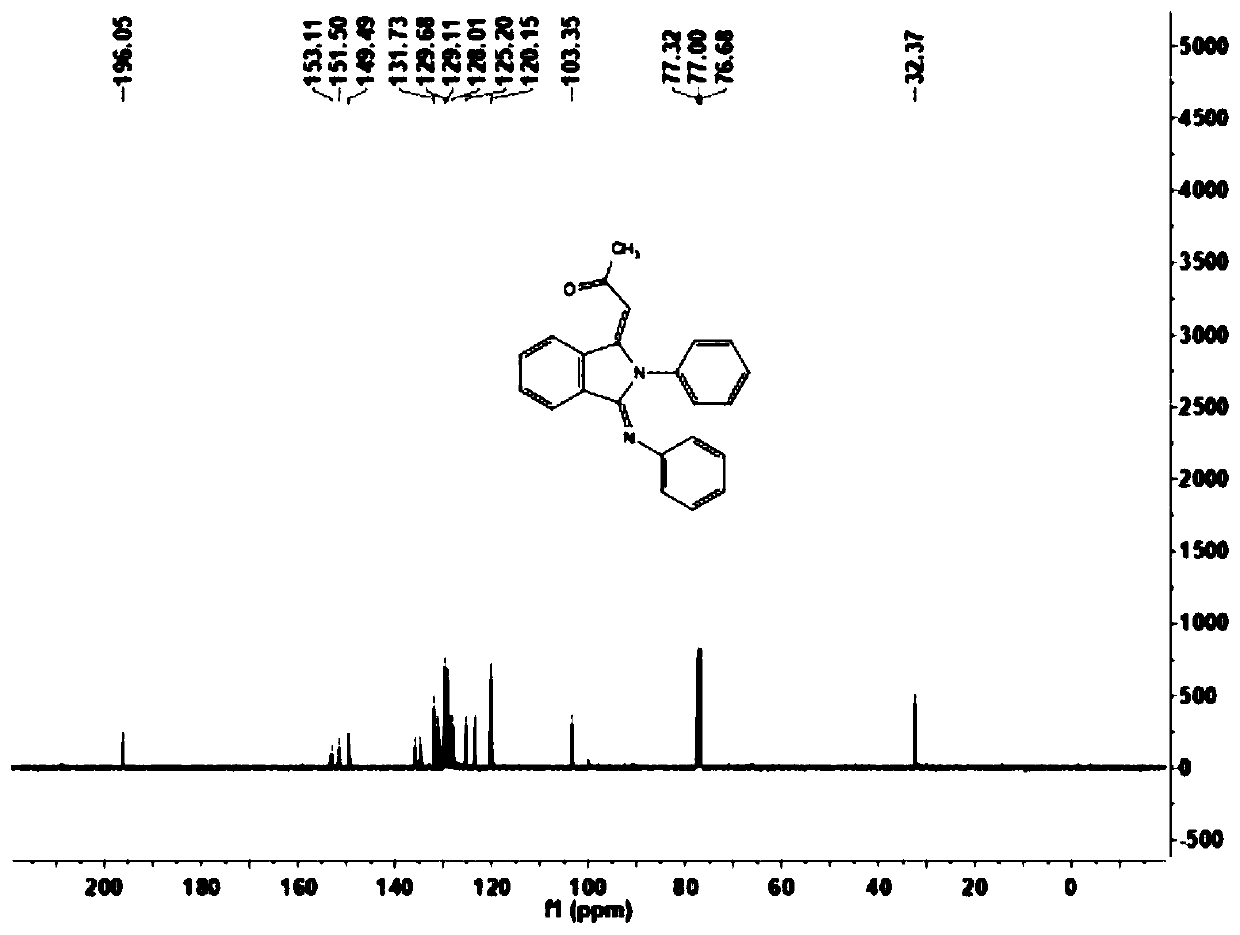
[0051] This example carries out the preparation of 1-((1E,3Z)-2-phenyl-3-(phenylamino)isoindoline-1-enyl-2-propanone (1a), and its reaction formula is as follows :
[0052]
[0053] Under the air atmosphere, the aromatic aldehyde compound 2a (25 mg, 0.2 mmol) shown in the formula II was sequentially added to the reactor, and the amine compound 3a (46 mg, 0.5 mmol) shown in the formula III was dissolved in 1,2-dichloroethane ( 0.5mL) was stirred at room temperature for one hour, and then 2mg of pentamethylcyclopentadienyl rhodium(III) chloride dimer, 5mg of bistrifluoromethanesulfonimide silver salt, 10mg of sodium acetate and 30mg of acetic acid were added successively Copper, use a syringe to inject a solution of terminal olefin 4a (20 μL, 0.20 mmol) in 1,2-dichloroethane (0.5 mL) into the reactor, place the reactor on the reaction device and adjust the temperature to 100 ° C, carry out After 12 hours of reaction, the end of the reaction was confirmed by thin-layer chroma...
Embodiment 2
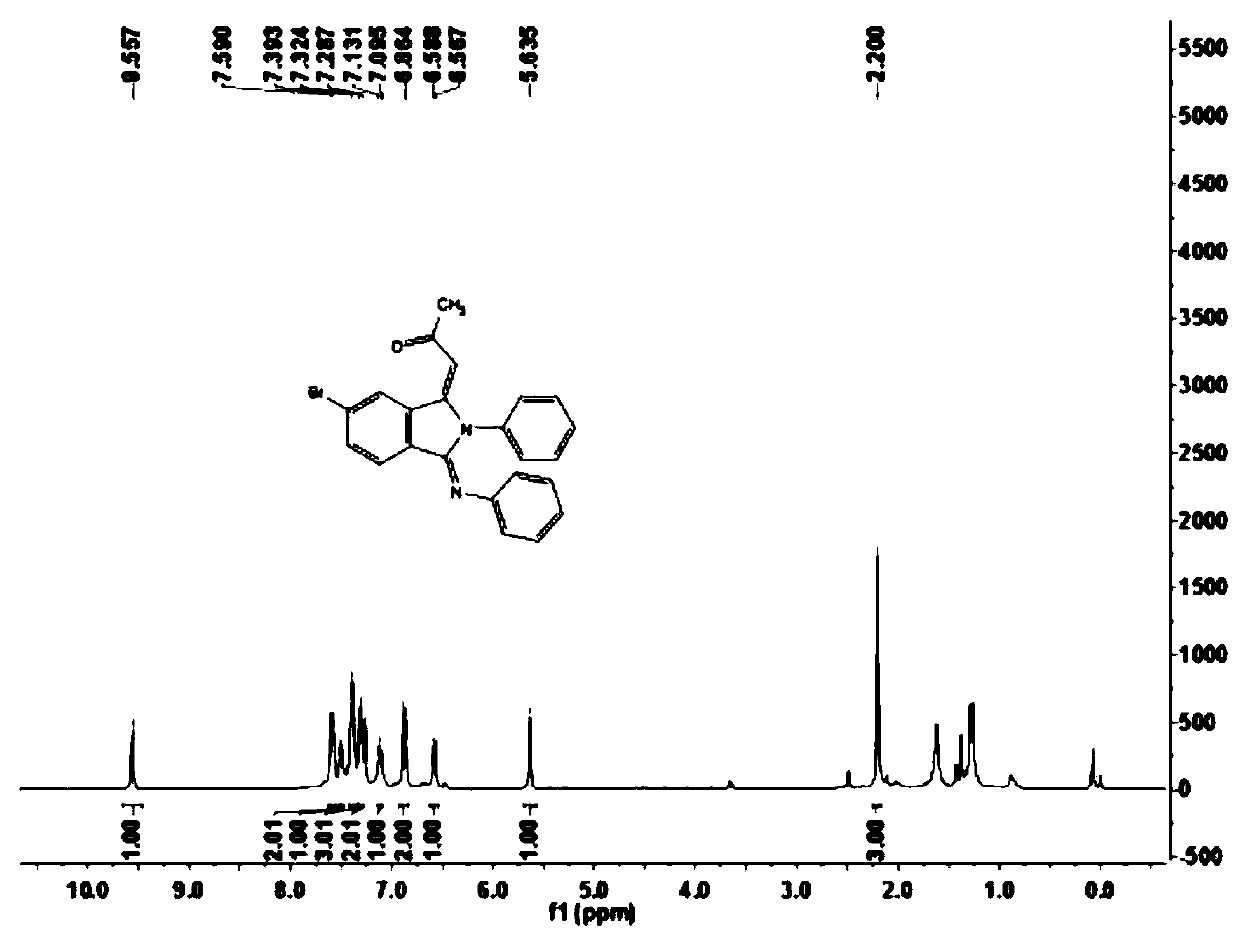
[0059] This embodiment carries out the preparation of 1-((1E,3Z)-6-bromo-2-phenyl-3-(phenylamino)isoindoline-1-enyl-2-propanone (1b), and its reaction The formula is as follows:
[0060]
[0061] Under the air atmosphere, the aromatic aldehyde compound 2b (37mg, 0.2mmol) shown in the formula II was sequentially added to the reactor, and the amine compound 3a (46mg, 0.5mmol) shown in the formula III was dissolved in 1,2-dichloroethane ( 0.5 mL) was stirred at room temperature for one hour, and then 2 mg of pentamethylcyclopentadienyl rhodium(III) chloride dimer, 5 mg of bistrifluoromethanesulfonimide silver salt, 20 mg of sodium acetate and 30 mg of acetic acid Copper, use a syringe to inject a solution of terminal olefin 4a (20 μL, 0.30 mmol) in 1,2-dichloroethane (0.5 mL) into the reactor, place the reactor on the reaction device and adjust the temperature to 100 ° C, carry out After 12 hours of reaction, the end of the reaction was confirmed by thin-layer chromatography ...
Embodiment 3
[0067] This example carries out the preparation of 1-((1E,3Z)-6-nitro-2-phenyl-3-(phenylamino)isoindoline-1-enyl-2-propanone (1c), which The reaction formula is as follows:
[0068]
[0069] Under the air atmosphere, the aromatic aldehyde compound 2c (30 mg, 0.2 mmol) shown in the formula II was sequentially added to the reactor, and the amine compound 3a (46 mg, 0.5 mmol) shown in the formula III was dissolved in 1,2-dichloroethane ( 0.5mL) solution was stirred at room temperature for one hour, then 2mg pentamethylcyclopentadienyl rhodium(III) chloride dimer, 5mg bistrifluoromethanesulfonimide silver salt, 10mg sodium acetate and 40mg acetic acid were added successively Copper, use a syringe to inject a solution of terminal olefin 4a (30 μL, 0.30 mmol) in 1,2-dichloroethane (0.5 mL) into the reactor, place the reactor on the reaction device and adjust the temperature to 100 ° C at the same time, carry out After 12 hours of reaction, the end of the reaction was confirmed by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com