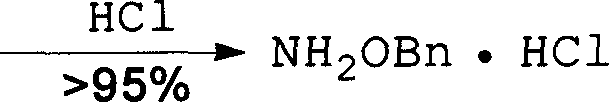
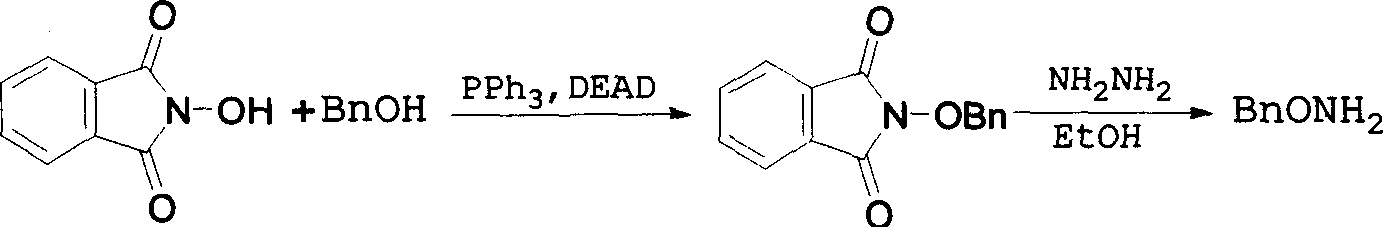
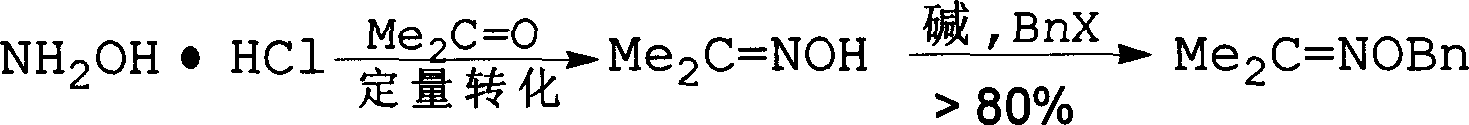Method for synthesizing benzyloxy amine hydrochloride
A technology for synthesizing benzyloxyamine hydrochloride and synthetic routes, which is applied in the field of synthesis of benzyloxyamine hydrochloride, can solve the problems of increased difficulty of synthesis, decreased reaction yield, high reagent cost, etc., and achieves low cost and mild conditions , The effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Step 1 Synthesis of free hydroxylamine
[0037]
[0038] Weigh hydroxylamine hydrochloride (403mg, 5.8mmol), add ethanol 1.3mL and stir at room temperature to completely dissolve hydroxylamine hydrochloride. Sodium hydroxide (725mg, 18.1mmol) aqueous solution (0.3mL) was slowly added in batches, and the reaction temperature was controlled at 25-40°C. After the addition of alkali, the reaction system was naturally stirred and cooled to room temperature for 0.5h. The next reaction was carried out directly without separation.
[0039] Step 2 synthesis of hydroxylamine acetone oxime 2
[0040]
[0041] Acetone (0.3mL) was added dropwise with a dropping funnel. The acetone was exothermic violently and cooled in an ice bath. Meanwhile, the reaction temperature was controlled at 0-60°C by controlling the rate of acetone addition. After the reaction was stable, it was reacted at room temperature for 15 hours. Extract with ether, wash the combined organic phase wi...
Embodiment 2
[0049] Step 1 Release free hydroxylamine
[0050] Hydroxylamine was prepared from compound 1 according to the method of Example 1.
[0051] Hydroxylamine hydrochloride (10.0 g, 144 mmol) was weighed in a double-necked flask equipped with a dropping funnel, 15 mL of water was added into the double-necked flask, and the hydroxylamine hydrochloride was completely dissolved by stirring at room temperature. Potassium hydroxide (8.9g, 158mmol) was slowly added in batches in an ice bath. After adding the base, the reaction temperature was controlled not to exceed 50°C. After 5h, the reaction system was cooled to room temperature, and the next reaction was carried out directly.
[0052] Step 2 synthesis of hydroxylamine acetone oxime 2
[0053] Hydroxyamine acetone oxime is prepared by the method of embodiment 1 by hydroxylamine.
[0054] Acetone (14mL) was added dropwise with a dropping funnel. When acetone was added, the heat was exothermic violently. The reaction temperature was ...
Embodiment 3
[0061] Step 1 Synthesis of free hydroxylamine
[0062] Hydroxylamine was prepared from compound 1 according to the method of Example 1.
[0063] Step 2 synthesis of hydroxylamine acetone oxime 2
[0064] Hydroxyamine acetone oxime is prepared by the method of embodiment 2 by hydroxylamine.
[0065] Step 3 Synthesis of benzyloxyamine acetone oxime 3
[0066] According to the method of Example 1, benzyloxyamine acetone oxime was prepared from compound 2.
[0067] Weigh NaH (1.9g, 82mmol), under nitrogen atmosphere, wash off the mineral oil on the surface of sodium hydrogen with dry tetrahydrofuran, add DMF (200mL) after washing, cool with ice-water bath, add dropwise hydroxylamine acetone oxime ( 5g, 68.5mmol) in DMF (50ml), continue to react at 0°C for 0.5h after the dropwise addition until there is no obvious bubbling. Benzyl bromide BnBr (8.2ml, 68.5mmol) was added dropwise in an ice-water bath, and the reaction continued for 5h after the addition was complete. Add satur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com