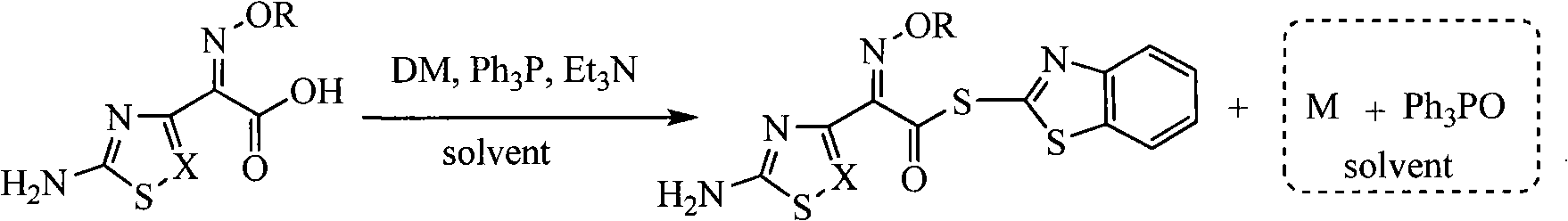
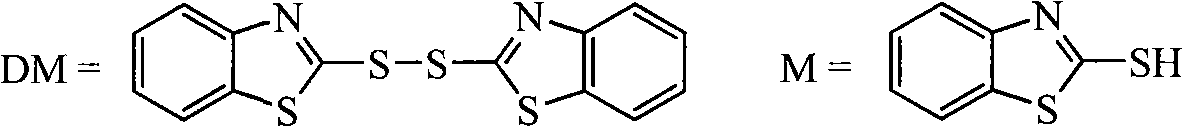
Process for recovering triphenyl phosphine oxide and 2-mercaptobenzothiazole from production waste liquid of cephalothin active ester
A technology for mercaptobenzothiazole and triphenylphosphine oxide, which is applied in the field of recovering triphenylphosphine oxide and 2-mercaptobenzothiazole, can solve the problems of poor product purity, high recovery cost, low recovery rate, etc. The effect of large implementation value and potential social and economic benefits, easy operation and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 reclaims the method for triphenylphosphine oxide and 2-mercaptobenzothiazole from ceftazidime active ester production waste liquid (theoretical amounts of TPPO and M are respectively 166.8g and 118.0g)
[0023] Add 4% sodium hydroxide aqueous solution dropwise at 10°C to 2L of waste liquid produced by ceftazidime side chain acid and dibenzothiazole disulfide under the action of triphenylphosphine, adjust the pH value to 10-11, and continue stirring after dropping React for 30 minutes, static layering. The organic layer was decompressed to recover the organic solvent, and the obtained solid waste residue weighed 250.0 g. Recrystallized with 125 g of toluene to obtain 155.0 g of white crystalline solid. The product was triphenylphosphine oxide. The rate is 92.9%. Heat the water layer to 65°C, use 12% hydrochloric acid to adjust the pH value of the system to 1.0-2.0, a white solid precipitates, filter while hot, wash with water, and dry to obtain 117.0 g of w...
Embodiment 2
[0024] Embodiment 2 reclaims the method for triphenylphosphine oxide and 2-mercaptobenzothiazole from ceftazidime active ester production waste liquid (theoretical amounts of TPPO and M are respectively 168.0g and 105.0g)
[0025] Add 4% sodium hydroxide aqueous solution dropwise to 2L of waste liquid produced by ceftazidime side chain acid and dibenzothiazole disulfide under the action of triphenylphosphine, adjust the pH value to 11-12 at 0°C, and continue stirring after dropping Reaction for 1.0 hour, static layering. The organic layer was decompressed to recover the organic solvent, and the obtained solid waste residue weighed 245.0 g. It was recrystallized with 2450 g of n-hexane to obtain 108.0 g of a white crystalline solid. The product was triphenylphosphine oxide. The recovery rate was 64.3%. Heat the water layer to 50°C, use 18% hydrochloric acid to adjust the pH of the system to 1.0-2.0, a white solid precipitates, filter while hot, wash with water, and dry to obta...
Embodiment 3
[0026] Embodiment 3 reclaims the method for triphenylphosphine oxide and 2-mercaptobenzothiazole from AE active ester production waste liquid (theoretical amounts of TPPO and M are respectively 165.0g and 95.0g)
[0027] Add 10% sodium hydroxide aqueous solution dropwise at 40°C to 2L waste liquid produced by aminothiaxamic acid and dibenzothiazole disulfide under the action of triphenylphosphine, adjust the pH value to 11-12, and continue stirring for 1.5 hours after dropping , static stratification. The organic layer was decompressed to recover the organic solvent, and the obtained solid waste residue weighed 256.0 g. It was recrystallized with 128 g of n-propanol to obtain 134.1 g of a white crystalline solid. , and the recovery rate was 81.3%. Heat the water layer to 40°C, use 15% hydrochloric acid to adjust the pH of the system to 1.0-2.0, a white solid precipitates, filter while hot, wash with water, and dry to obtain 93.0 g of white powder solid, the product is 2-merca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com