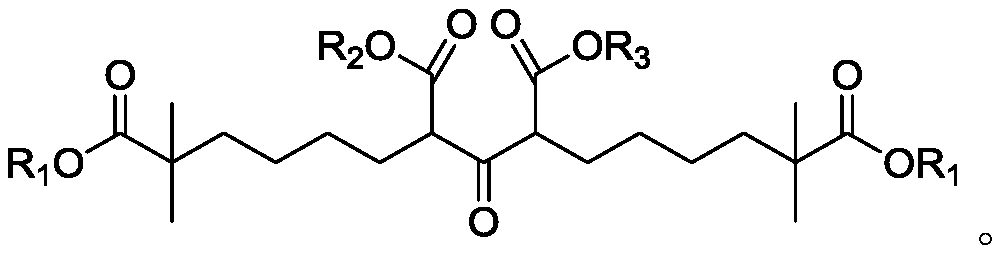
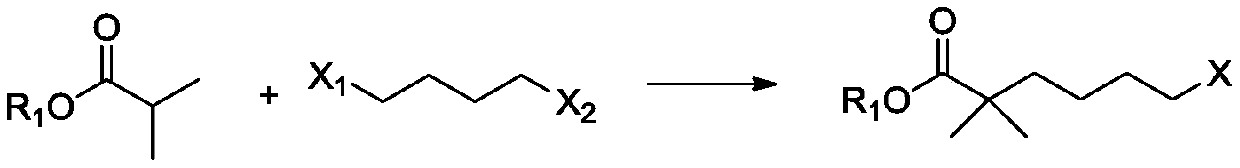Compound and method for synthesizing 8-hydroxy-2,2,14,14-tetramethyl pentadecanedioic acid by adopting compound
A technology of tetramethylpentadecane and compounds, which is applied in the field of chemical synthesis, can solve problems such as difficulty in guaranteeing product quality and impracticability, and achieve the effect of short steps and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Add 300g (2.58mol) of ethyl isobutyrate and 724.9g (3.36mol, 1.3eq) of 1,4-dibromobutane into 1500ml of tetrahydrofuran, start stirring, cool down to -10~0℃, and control the temperature to -10~ Add 1.36L lithium diisopropylamide (2N, 2.71mol, 1.05eq.) dropwise at 10°C. After dropping, GC shows that the conversion rate is 76.14%. Add 100ml of water to quench the reaction, and use 3N HCl to adjust the pH to 6-7 (980ml ), liquid separation, the organic phase was washed with 600ml*2 saturated sodium chloride, concentrated, rectified, and vacuum degree of 180Pa was collected, and the fraction at 82-87°C was obtained to obtain 423.7g of 2,2-dimethyl-6-bromohexanoic acid Ethyl ester, yield: 65.3%.
[0072] Other examples are described below, and the differences from Embodiment 1 are shown in the table below:
[0073]
[0074]
[0075] Second and third steps
[0076]
Embodiment 24
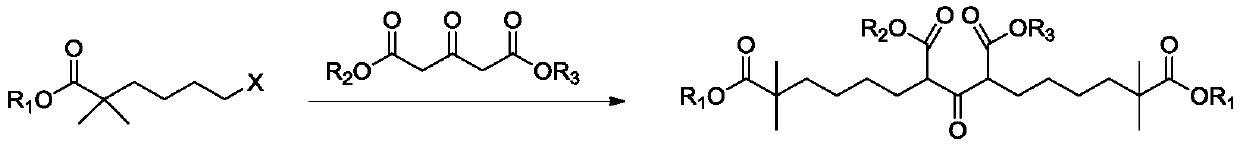
[0078] Add 60g (239mmol) ethyl 2,2-dimethyl-6-bromohexanoate to 300ml ethanol, and add 26.6g (131mmol) diethyl 1,3-acetonedicarboxylate, 49.5g (359mmol ) Potassium carbonate, 39.7g (239mmol) potassium iodide, heat up to 65-70°C, keep warm for 20 hours, cool the system to room temperature, filter, rinse the filter cake with 50ml ethanol, add 300ml purified water to the mother liquor, add 43g (1075mmol) Sodium hydroxide, heated to reflux, kept reflux for 4 hours, after NMR traced the reaction, concentrated the system to dryness, added 400ml water to dissolve, extracted with 200ml methyl tert-butyl ether after dissolution, after liquid separation, used 36% concentrated hydrochloric acid Adjust the pH of the water phase to 1-2, control the temperature of the system at 0-10°C, stir for 15 minutes, filter, dissolve the filter cake with 300ml of methyl tert-butyl ether, wash with 200ml of purified water, and concentrate the upper organic phase to dryness after liquid separation Using...
Embodiment 25
[0080] Add 30g (119mmol) ethyl 2,2-dimethyl-6-bromohexanoate into 200ml tetrahydrofuran, and add 12.08g (59.7mmol) diethyl 1,3-acetonedicarboxylate successively under stirring, 19g (137.5 mmol) potassium carbonate, 2g (12.0mmol) potassium iodide, warming up to reflux, after insulation for 100 hours, the system was cooled to room temperature, filtered, filter cake was rinsed with 20ml tetrahydrofuran, after the mother liquor was concentrated, 90ml ethanol was added, 120ml purified water, 21.3g (535mmol) sodium hydroxide, heated to reflux, kept reflux for 4 hours, after the NMR tracking reaction was completed, the system was concentrated to dryness, added 80ml of water to dissolve, extracted with 100ml methyl tert-butyl ether after dissolving, after liquid separation, Use 36% concentrated hydrochloric acid to adjust the pH of the water phase to 1-2, control the temperature of the system at 0-10°C throughout the process, stir for 15 minutes, filter, and recrystallize the filter ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com