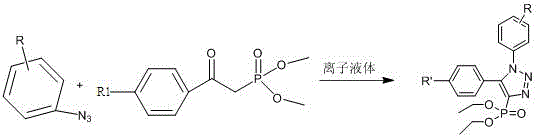
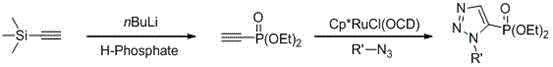Method for synthesizing 4-phosphonic acid-1,5-substituted-1,2,3 triazole compounds through catalysis of ionic liquid
An ionic liquid and compound technology, applied in the field of synthesis of triazole compounds, can solve the problems of limited synthesis methods, limited substrate expansion, inability to introduce substituents, etc., and achieves improved bioavailability, improved water solubility and atom utilization. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] First add 3-trifluoromethylphenyl azide (0.1mmol) to the round bottom flask, then add ionic liquid TMGAc (0.15mmol), stir evenly, then add acetonylphosphonic acid dimethyl ester (0.1mmol), Stirring at 50°C for 1.5h, the whole reaction process was detected by TLC until the reaction was completed. Finally, the crude product was extracted with water and ethyl acetate, dried with anhydrous magnesium sulfate, and then separated by silica gel column to obtain the pure product with a yield of 95%.
Embodiment 2
[0021] Add 4-bromophenyl azide (0.1mmol) to the round bottom flask first, then add ionic liquid TMGOH (0.15mmol), stir evenly, then add dimethyl acetonyl phosphonate (0.1mmol), stir at 50°C The reaction was carried out for 2 h, and the whole reaction process was detected by TLC until the reaction was completed. Finally, the crude product was extracted with water and ethyl acetate, dried with anhydrous magnesium sulfate, and then separated by silica gel column to obtain the pure product with a yield of 95%.
Embodiment 3
[0023] First add 4-methoxyphenyl azide (0.1mmol) to the round bottom flask, then add ionic liquid CHLac (0.15mmol), stir evenly, then add dimethyl acetonyl phosphonate (0.1mmol), 50 The reaction was stirred at ℃ for 2.5 h, and the whole reaction process was detected by TLC until the reaction was completed. Finally, the crude product was extracted with water and ethyl acetate, dried with anhydrous magnesium sulfate, and then separated by silica gel column to obtain the pure product with a yield of 54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com