Intermediate compound, and synthetic method of prothioconazole
A technology of prothioconazole and synthetic method, which is applied in the field of organic synthesis, can solve problems such as yield decline, and achieve the effects of avoiding post-processing, high conversion rate and selectivity, and cheap and easy-to-obtain synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of 2-(1-chlorocyclopropyl)-3-chloro-1-(2-chlorophenyl)-2-propanol
[0051] In a 250mL round-bottomed three-neck flask device with a constant pressure titration funnel and a condenser tube, anhydrous and anaerobic operations were carried out under nitrogen protection conditions, and 10g (0.062mol) of 2-chlorobenzyl and 50mL of THF solution were mixed and placed in a constant pressure drop In the funnel, add 1.8g (0.074mol) of magnesium chips, 10ml of THF solution and a small amount of iodine into the three-necked flask, drop in 2ml of THF solution of 2-chlorobenzyl chloride, trigger with slight heat, put the device into the ice bath and slowly Add dropwise (drop / 3s) until the drop is over, and then react for 1 h after the drop is over, slowly drop the above Grignard reactant into THF (30mL) of 9.0g (0.058mol) 1-chloro-1-chloroacetylcyclopropane The solution was slowly added dropwise (drop / 3s) until the drop was completed, and the reaction was continued for 1 ho...
Embodiment 2
[0053] Synthesis of Prothioconazole
[0054] Synthesis of compound (Ⅳ)
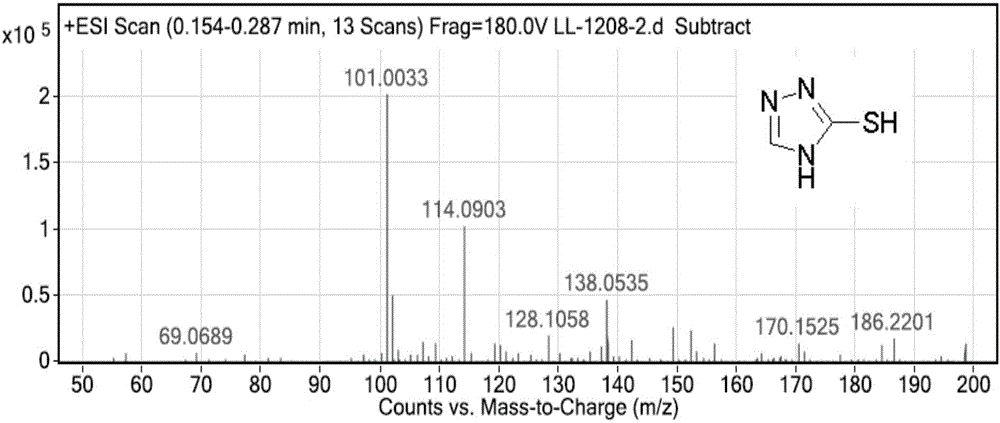
[0055] Dissolve 5g (0.072mol) of 1,2,4-triazole in 15ml of DMF, add 6.9g (0.216mol) of sublimed sulfur, heat to 140°C, react for 3.5h, cool to room temperature, filter, and use the filtrate Wash with saturated sodium chloride, extract with ethyl acetate, separate the organic phase, dry over anhydrous sodium sulfate, distill off the ethyl acetate to obtain 6.95 g of solid compound (IV), with a yield of 95%.
[0056] Synthesis of compound (Ⅴ)
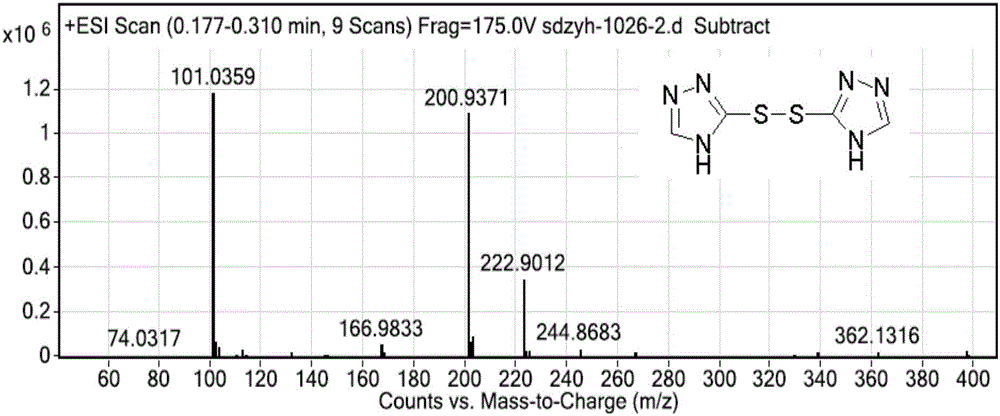
[0057] 3.03g (30mmol) of compound (Ⅳ) was dissolved in 30mL DCM, and 2.37g (30mmol) of redistilled pyridine was added. Under ice-bath stirring conditions, 2.64g (15mmol) benzenesulfonyl chloride was added dropwise, and the dropwise addition was completed in about 1 hour. Remove the ice-water bath and stir at room temperature for 5h. DCM was evaporated, and 15 mL of water and 10 mL of ethyl acetate were added to the residue, reacted for 1 h, filtered, and washed w...
Embodiment 3
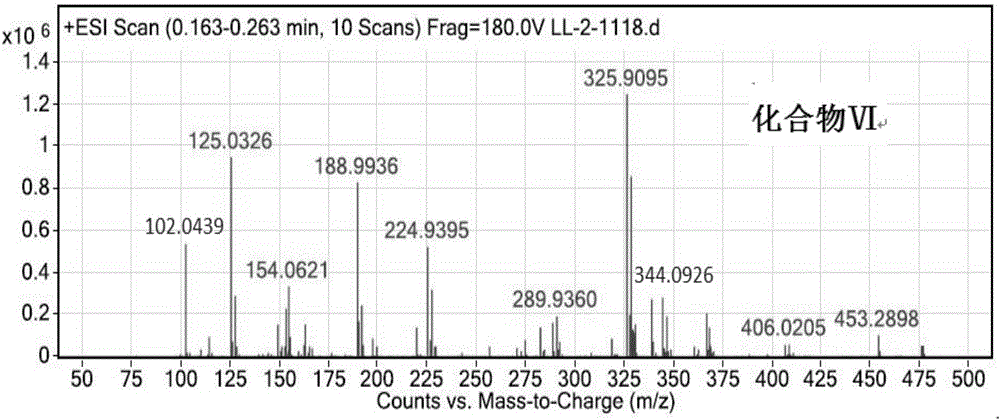
[0085] Synthesis of compound (Ⅵ)
[0086] Stir and mix 6g (30mmol) of compound (Ⅴ), 30ml of DMF, and 12.42g (90mmol) of potassium carbonate, and heat to 60°C. 17.61 g (63 mmol) of compound (II) was added dropwise, and the dropwise addition was completed after 2 hours. The reaction was continued, stopped, and cooled to room temperature. Suction filtration, the filtrate was washed with saturated brine, extracted with ethyl acetate, the organic phase was washed three times with saturated brine, the organic phase was separated, dried over anhydrous sodium sulfate, and the product obtained by rotary evaporation. 17.31 g of compound (VI) was obtained with a yield of 84%.
[0087] Synthesis of Target Compound (Ⅶ) Prothioconazole
[0088] Dissolve 6.25g (0.009mol) of compound (Ⅵ) in 30ml of methanol, add 1.21g (0.019mol) of metal Zn, stir and react at 40°C for 3h, stop the reaction, directly recrystallize, precipitate the crystals and filter to obtain the target compound (Ⅶ) Prothi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com