Bisbenzothiazole disulfide and triphenylphosphine preparation by means of one pot
A technology for benzothiazole disulfide and triphenylphosphine, which is applied in the field of preparing dibenzothiazole disulfide and triphenylphosphine, can solve the problems of unsatisfactory methods, high reaction temperature, unfriendly environment, and the like, To achieve the effects of cheap reagents, high reaction yield and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
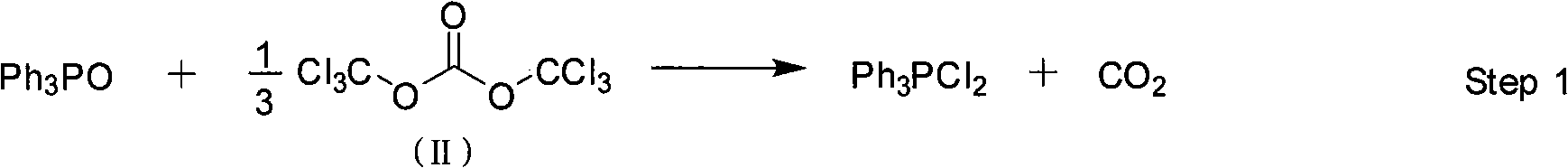
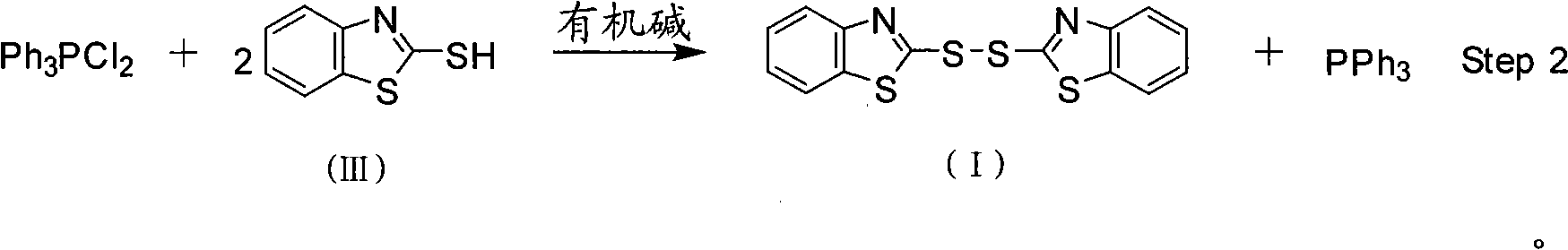
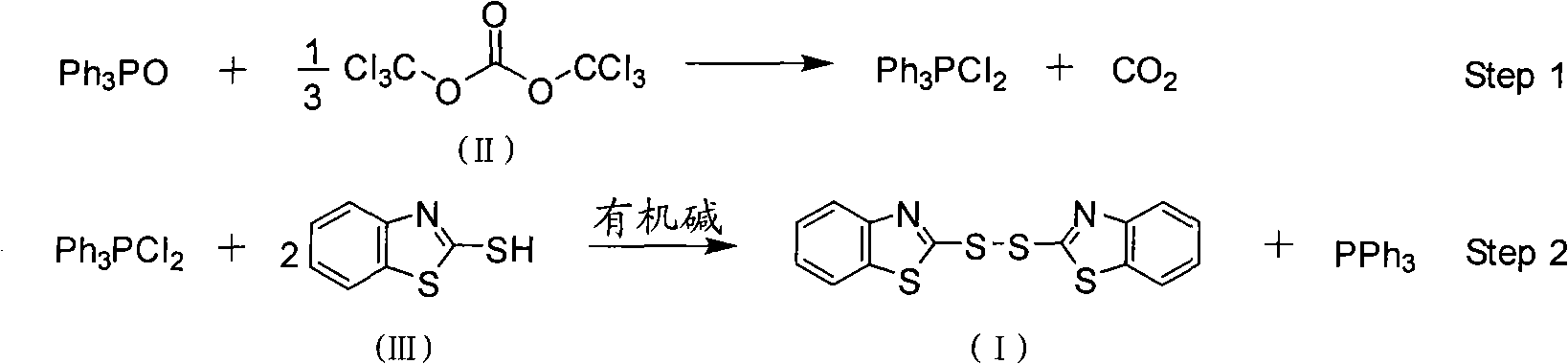
[0028] Feeding ratio is TPPO: BTC: M: organic base=1.0: 0.34: 2.0: 1.5, organic base is triethylamine, organic solvent A and B are chlorobenzene, organic solvent A in step (1) and step (2) The dosage of B and B is respectively 4 times and 2 times of TPPO feeding quality. The reaction temperature in the first stage is 20°C (T 1 ), reaction time 5h(t 1 ), the reaction temperature in the second stage is 20°C (T 2 ), reaction time 4h(t 2 ). The recrystallization solvent is isopropanol.
[0029] (1) Put chlorobenzene 111.2g, BTC (30.3g, 0.034mol), TPPO (27.8g, 0.1mol) into a 500mL four-necked flask equipped with a thermometer, dropping funnel, and mechanical stirring at 20°C, and keep warm for the reaction For 5 hours, the resulting reaction solution was directly used in step (2);
[0030] (2) At 20°C, add dropwise a mixed solution of chlorobenzene (55.6g) that is dissolved with M (33.4g, 0.2mol), triethylamine (15.2g, 0.15mol) in the reaction solution obtained in step (1), ...
Embodiment 2
[0033] Feeding ratio is TPPO: BTC: M: organic base=1.0: 0.4: 2.0: 2.0, organic base is pyridine, organic solvent A and B are toluene, organic solvent A and B in step (1) and step (2) The dosage is 3 times and 5 times of the mass of TPPO feeding respectively. T 1 =30°C, t 1 = 3h; T 2 =20°C, t 2 = 6h. The recrystallization solvent is isopropanol.
[0034] Others are the same as in Example 1, the product DM yield is 92.6%, HPLC purity 99.0%; TPP yield 91.0%, HPLC purity 98.8%.
Embodiment 3
[0036]Feeding ratio is TPPO: BTC: M: organic base=1.0: 0.34: 1.5: 1.5, organic base is triethylamine, organic solvent A is acetonitrile, and organic solvent B is chlorobenzene, in step (1) and step (2) The dosages of organic solvents A and B were 1 and 2 times the mass of TPPO feed, respectively. T 1 = 0°C, t 1 =10h;T 2 =90°C, t 1 = 2h. The recrystallization solvent is ethanol.
[0037] Others are the same as in Example 1, the product DM yield is 73.2%, and the HPLC purity is 99.3%; the TPP yield is 71.9%, and the HPLC purity is 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com