3-amino indanone compound synthesis method
A technology of aminoindanone and synthesis method, which is applied in the direction of preparation of organic compounds, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., to achieve wide substrate adaptability, high conversion efficiency, and reaction yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0035] Add imine (0.2mmol), olefin compound (0.4mmol), pentamethylcyclopentadiene rhodium dichloride (0.02mmol), manganese acetate (0.2mmol) and 1ml of organic solvent, mixed and stirred evenly, after the reaction was completed according to the reaction conditions in Table 2, cooling, suction filtration, silica gel mixing sample, purified by column chromatography to obtain the corresponding 3-aminoindanone compound (I), the reaction process is as follows Show:
[0036]
[0037] Table 1 Raw material ratio of Examples 1-11
[0038]
[0039]
[0040] Table 2 Reaction conditions and reaction results of Examples 1 to 11
[0041]
[0042] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, n-Bu is n-butyl, 2-naphthyl is 2-substituted naphthyl, CF 3 is trifluoromethyl, Ph is phenyl, and 2-thiophene is 2-substituted thiophene.
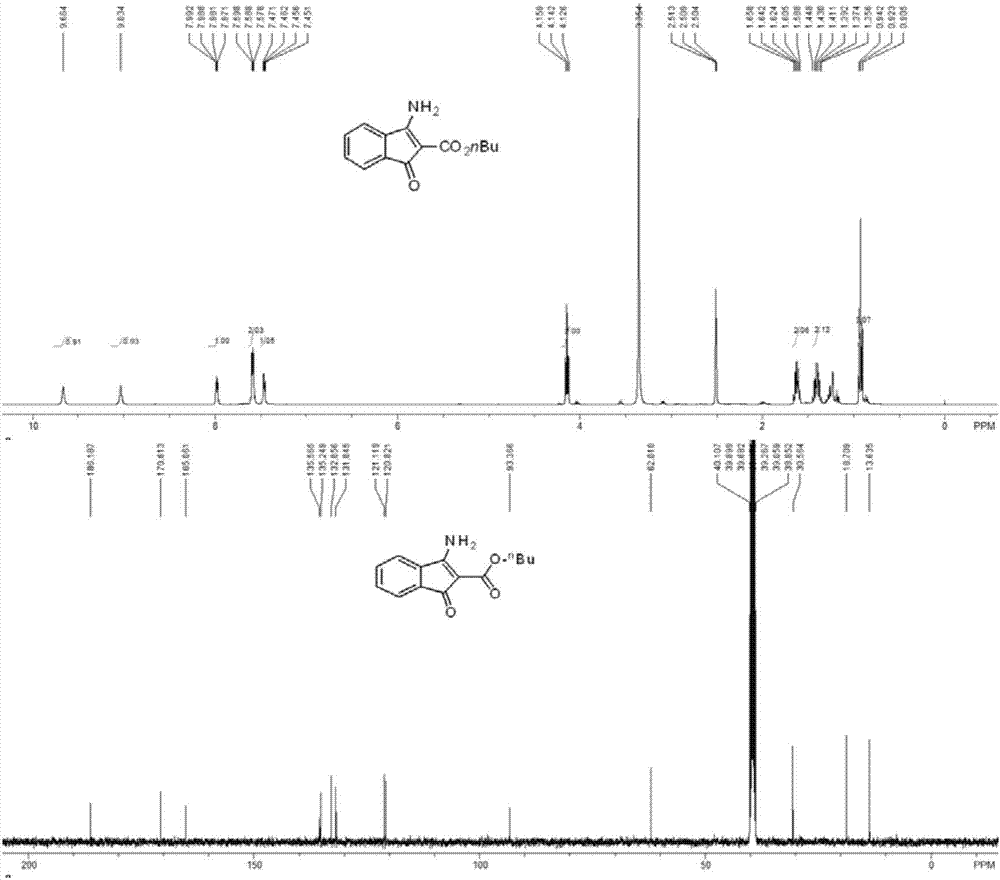
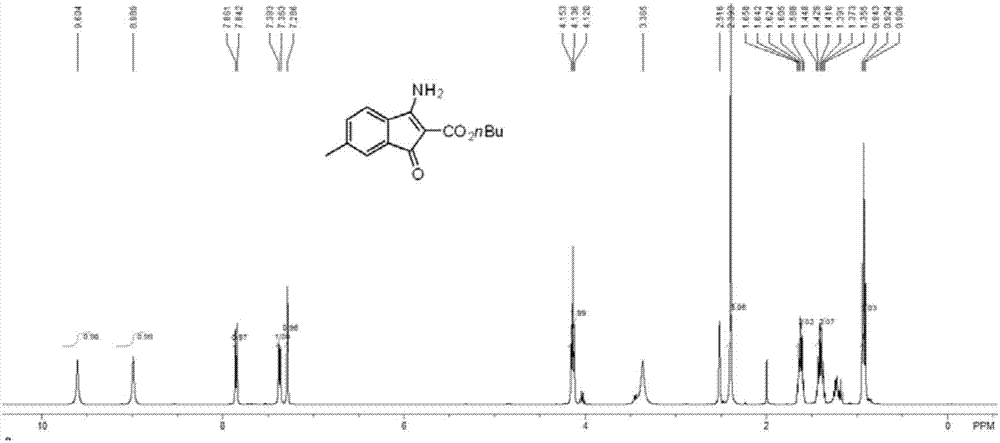
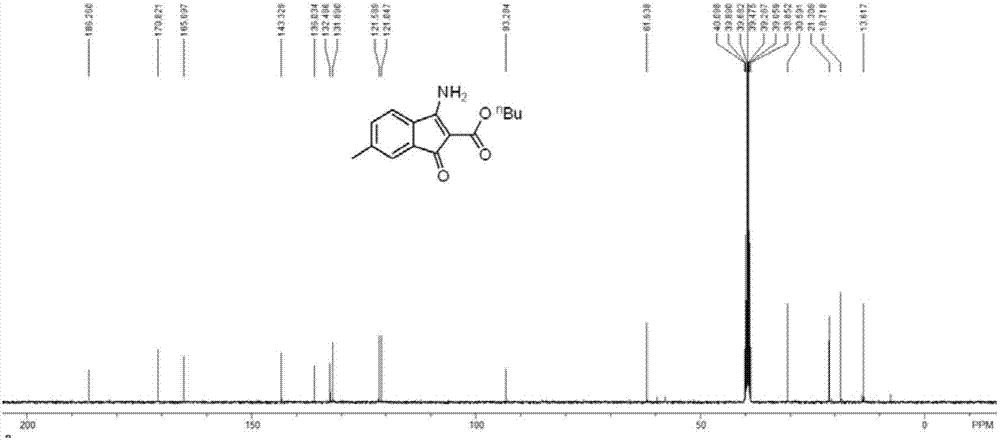
[0043] The structure confirmation data of some compounds prepared in Examples 1-10:
[0044]
[0045] butyl 3-am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com