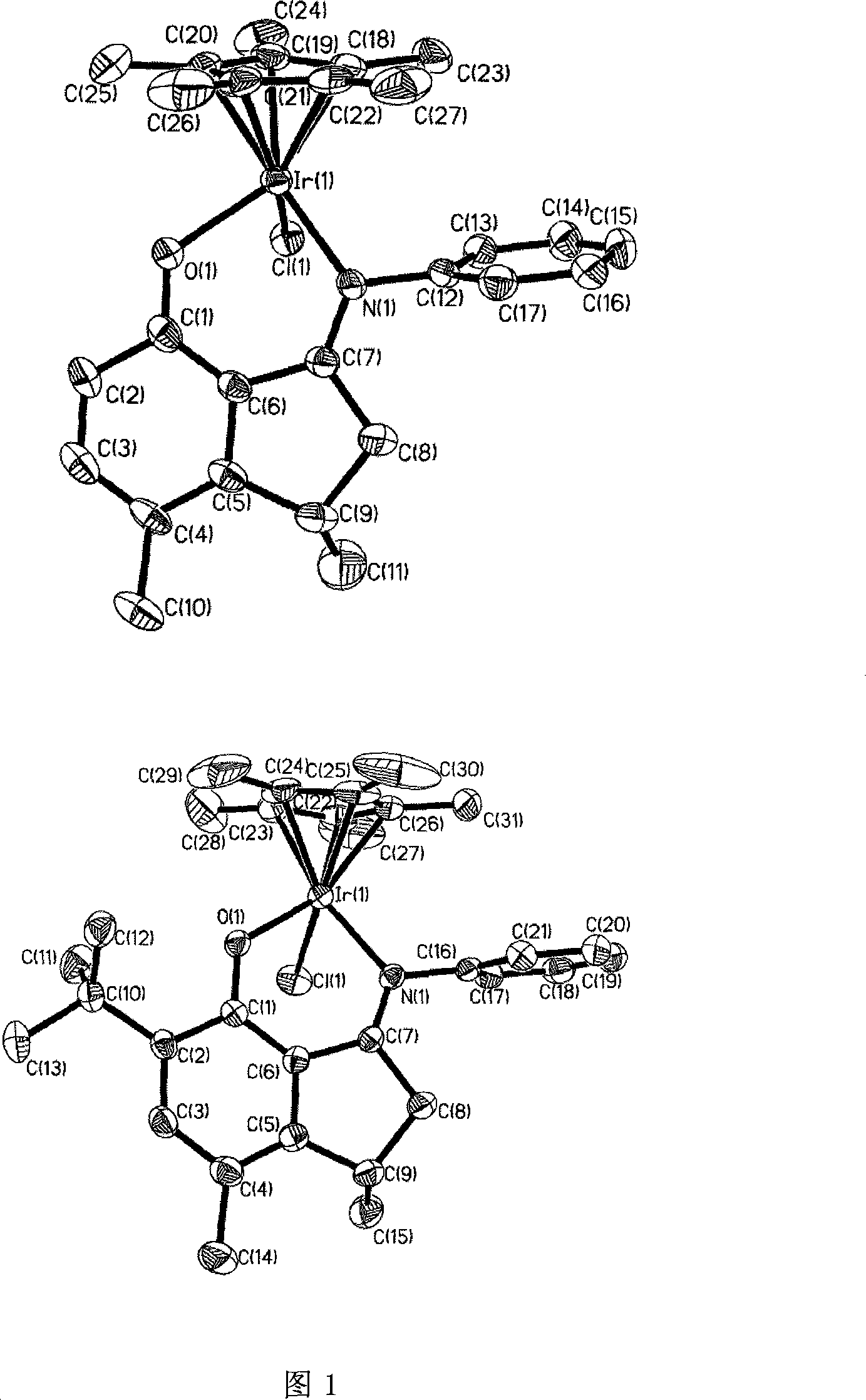
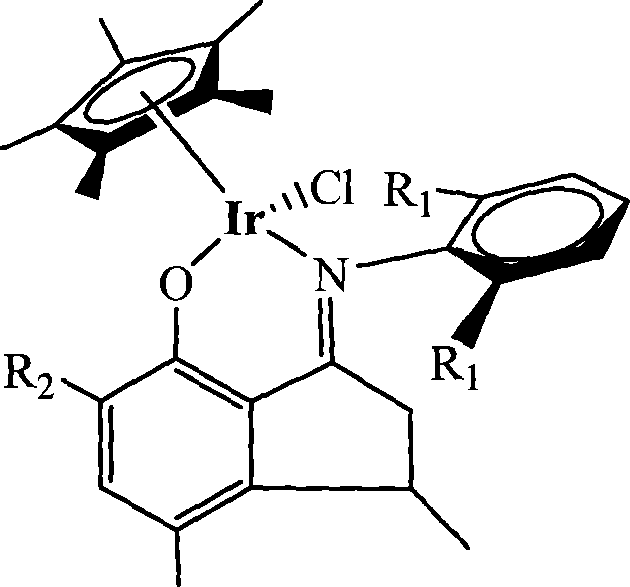
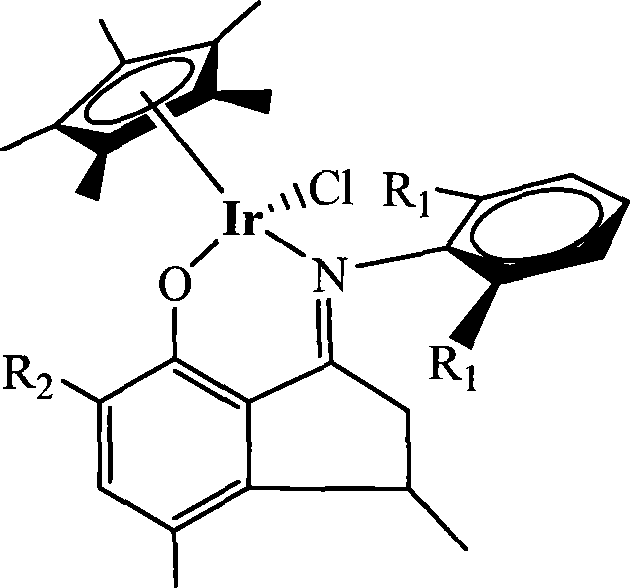
Method for preparing norbornene open-loop translocation polymer or addition polymer
A technology of norbornene and ring-opening metathesis, applied in the field of chemical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Ligand (C 6 h5 )N=CC 2 h 3 (CH 3 )C 6 h 2 (CH 3 ) Preparation of OH
[0033] in N 2 2.0g (11.4mmol) 7-hydroxy-3,4-dimethylindanone and 1.4mL (15.0mmol) aniline were added to a 100mL three-necked flask, then 30mL absolute ethanol, 0.1mL formic acid, 0.7 g 4A molecular sieve. Heat to reflux for 48 hours. After the end of the reaction, filter, collect the filtrate, and use anhydrous Mg 2 SO 4 It was dried, concentrated, and passed through a silica gel column with a mixed solution of petroleum ether and ethyl acetate as an eluent to obtain 2.3 g of a yellow solid with a yield of 80%.
Embodiment 2
[0034] Example 2 Ligand [C 6 h 3 -i-(C 3 h 7 ) 2 -2,6] N=CC 2 h 3 (CH 3 )C 6 h 2 (CH 3 ) Preparation of OH
[0035] in N 2 1.3mL (11.4mmol) TiCl will be dissolved within 30min 4 30 mL of toluene solution was added to 40 mL of toluene solution containing 8.6 mL (45.4 mmol) of 2,6-diisopropylaniline. The reaction mixture was then heated to 90° C., and 2 g (11.4 mmol) of 7-hydroxy-3,4-dimethylindanone was added after 30 min. At this temperature, the reaction was carried out for 48 hours. After the reaction, the reaction solution was poured into Na 2 CO 3 Saturated aqueous solution, liquid separation, organic phase with anhydrous Mg 2 SO 4 It was dried, concentrated, and passed through a silica gel column using a mixed solution of petroleum ether and ethyl acetate as an eluent to obtain 1.54 g of a red solid with a yield of 40%.
Embodiment 3
[0036] Example 3 Ligand (C 6 h 5 )N=CC 2 h 3 (CH 3 )C 6 h 2 (CH 3 )(C 4 h 9 ) Preparation of OH
[0037] in N 2 1.3mL (11.4mmol) TiCl will be dissolved within 30min 4 30mL of toluene solution was added to 40mL of toluene solution containing 4.3mL (45.4mmol) aniline. Then the reaction mixture was heated to 90° C., and 2.63 g (11.4 mmol) of 7-hydroxy-3,4-dimethyl-6-tert-butylindanone was added after 30 min. At this temperature, the reaction was carried out for 48 hours. After the reaction, the reaction solution was poured into Na 2 CO 3 Saturated aqueous solution, liquid separation, organic phase with anhydrous Mg 2 SO 4 It was dried, concentrated, and passed through a silica gel column using a mixed solution of petroleum ether and ethyl acetate as an eluent to obtain 2.75 g of an orange-red solid with a yield of 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com