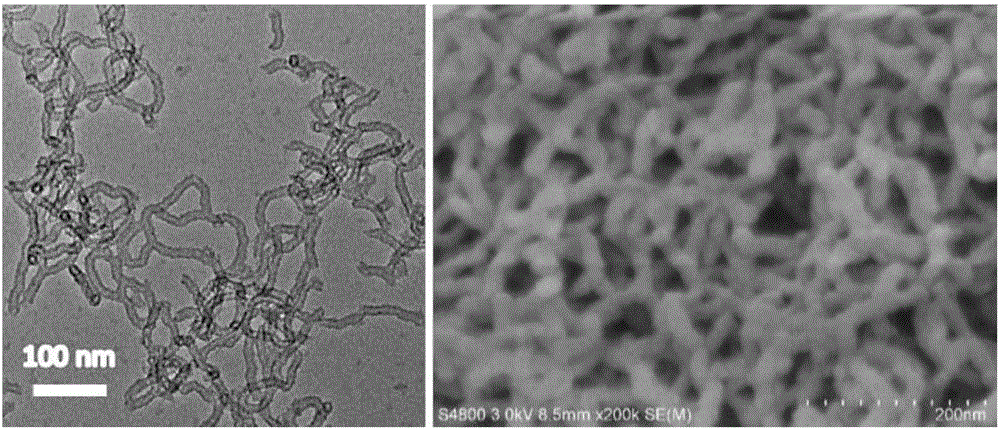
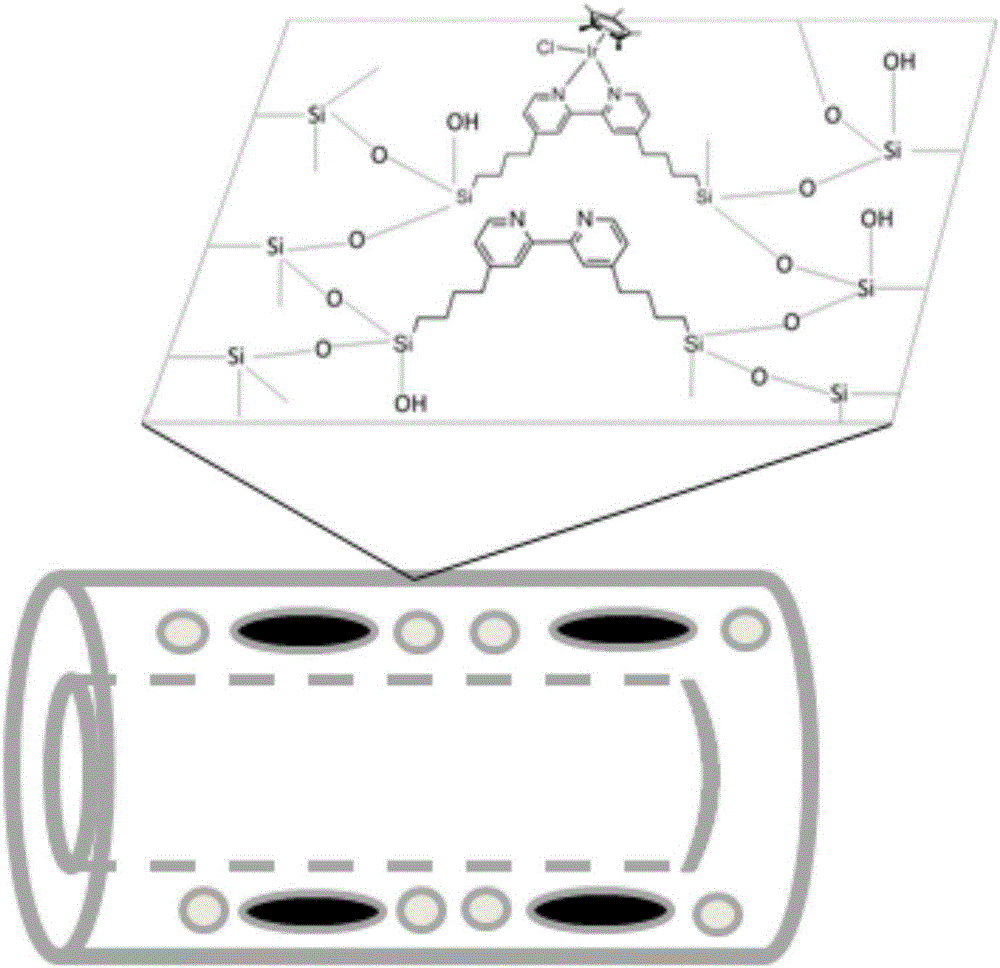
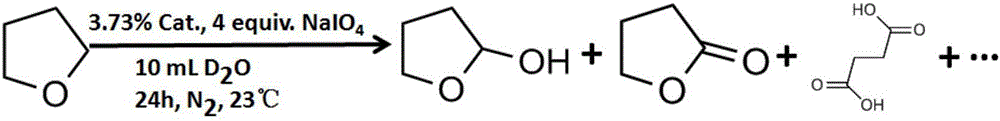
Tetrahydrofuran C-H multiphase oxidation method
A technology of tetrahydrofuran and potassium chloride, applied in the application field of organic matter conversion, can solve problems such as loss of activity, waste of catalyst, unstable catalyst, etc., and achieve the effects of mild reaction conditions and simple and easy preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1: Dissolve 0.875g KCl and 0.275g triblock copolymer surfactant polyoxyethylene-polyoxypropylene-polyoxyethylene EO20-PO70-EO20 (P123) in 120mL of 1.8M hydrochloric acid solution, stir rapidly to make Form a uniform transparent solution, add 3.15mmol organosilane precursor 1,4-bis(triethoxysilyl)benzene and continue to stir vigorously, reduce the stirring speed and continue stirring, then add 0.35mmol bipyridine precursor 4,4'-[4 -(Trimethoxysilanyl)butyl]-2,2'-bipyridine was stirred at 30°C for 24h.
[0034] Step 2: Add the obtained white emulsion into a 100ml polytetrafluoroethylene liner and place it in a constant temperature drying oven at 70°C. After standing for 30 hours, filter it with a sand core funnel, and wash it with deionized water several times to ensure that the filtrate is No more air bubbles are produced. After the obtained product was dried and dehydrated at 20°C, it was extracted with ethanol and concentrated hydrochloric acid at 60°C (the extra...
Embodiment 2
[0038]Step 1: Dissolve 1.75g KCl and 0.55g triblock copolymer surfactant polyoxyethylene-polyoxypropylene-polyoxyethylene EO20-PO70-EO20 (P123) in 120mL 2M hydrochloric acid solution, stir rapidly to form Uniform and transparent solution, add 2.8mmol organosilane precursor 1,4-bis(triethoxysilyl)benzene and continue to stir vigorously, reduce the stirring speed and continue stirring, then add 0.7mmol bipyridine precursor 4,4'-[4 -(Trimethoxysilanyl)butyl]-2,2'-bipyridine was stirred at 35°C for 18h.
[0039] Step 2: Add the obtained white emulsion into a 100ml polytetrafluoroethylene liner and place it in a 90°C constant temperature drying oven. After standing for 25 hours, filter it with a sand core funnel, and wash it with deionized water several times to ensure that the filtrate No more air bubbles are produced. After the obtained product was dried and dehydrated at 50°C, it was extracted and refluxed at 70°C for 8 hours using ethanol and concentrated hydrochloric acid. ...
Embodiment 3
[0043] Step 1: Dissolve 3.5KCl and 1.1g triblock copolymer surfactant polyoxyethylene-polyoxypropylene-polyoxyethylene EO20-PO70-EO20 (P123) in 120mL of 2.2M hydrochloric acid solution, stir rapidly to make it Form a uniform transparent solution, add 2.45mmol organosilane precursor 1,4-bis(triethoxysilyl)benzene and continue to stir vigorously, reduce the stirring speed and continue stirring, then add 1.05mmol bipyridine precursor 4,4'-[ 4-(Trimethoxysilanyl)butyl]-2,2'-bipyridine was stirred at 40°C for 12h.
[0044] Step 2: Add the obtained white emulsion into a 100ml polytetrafluoroethylene liner, place it in a constant temperature drying oven at 110°C, let it stand for 20 hours, filter it with a sand core funnel, and wash it with deionized water several times to ensure that the filtrate does not contain Bubbles are generated again. After the obtained product was dried and dehydrated at 60° C., extraction and reflux were carried out at 80° C. for 6 h using ethanol and conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Tube wall thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com