Patents
Literature
54 results about "Carbon–hydrogen bond activation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carbon–hydrogen bond functionalization (C–H functionalization) is a type of reaction in which a carbon–hydrogen bond is cleaved and replaced with a carbon-X bond (where X is usually carbon, oxygen, or nitrogen). The term usually implies that a transition metal is involved in the C-H cleavage process. Reactions classified by the term typically involve the hydrocarbon first to react with a metal catalyst to create an organometallic complex in which the hydrocarbon is coordinated to the inner-sphere of a metal, either via an intermediate "alkane or arene complex" or as a transition state leading to a "M−C" intermediate. The intermediate of this first step (known as C-H activation and sometimes used interchangeably with C-H functionalization) can then undergo subsequent reactions to produce the functionalized product. Important to this definition is the requirement that during the C–H cleavage event, the hydrocarbyl species remains associated in the inner-sphere and under the influence of "M".
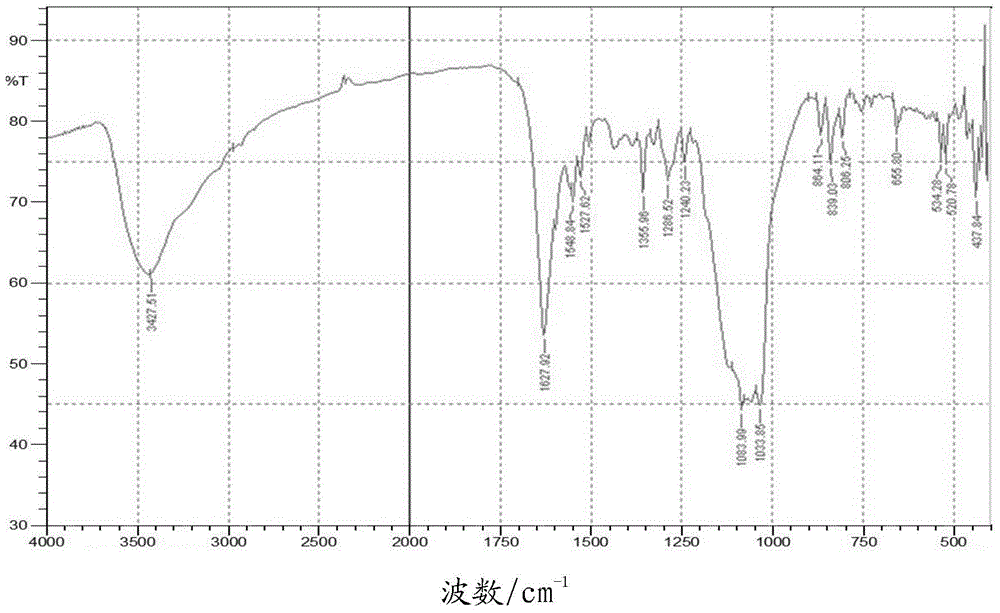
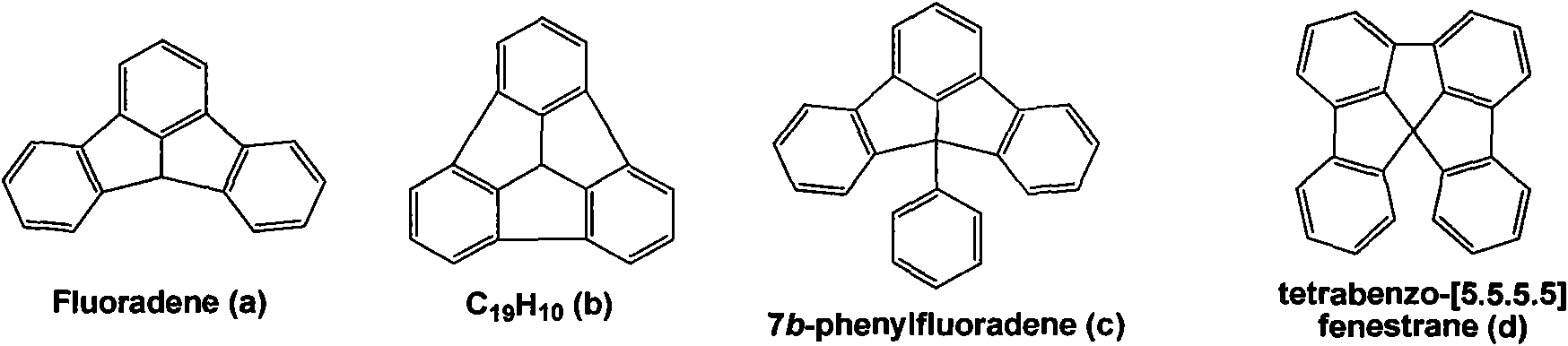
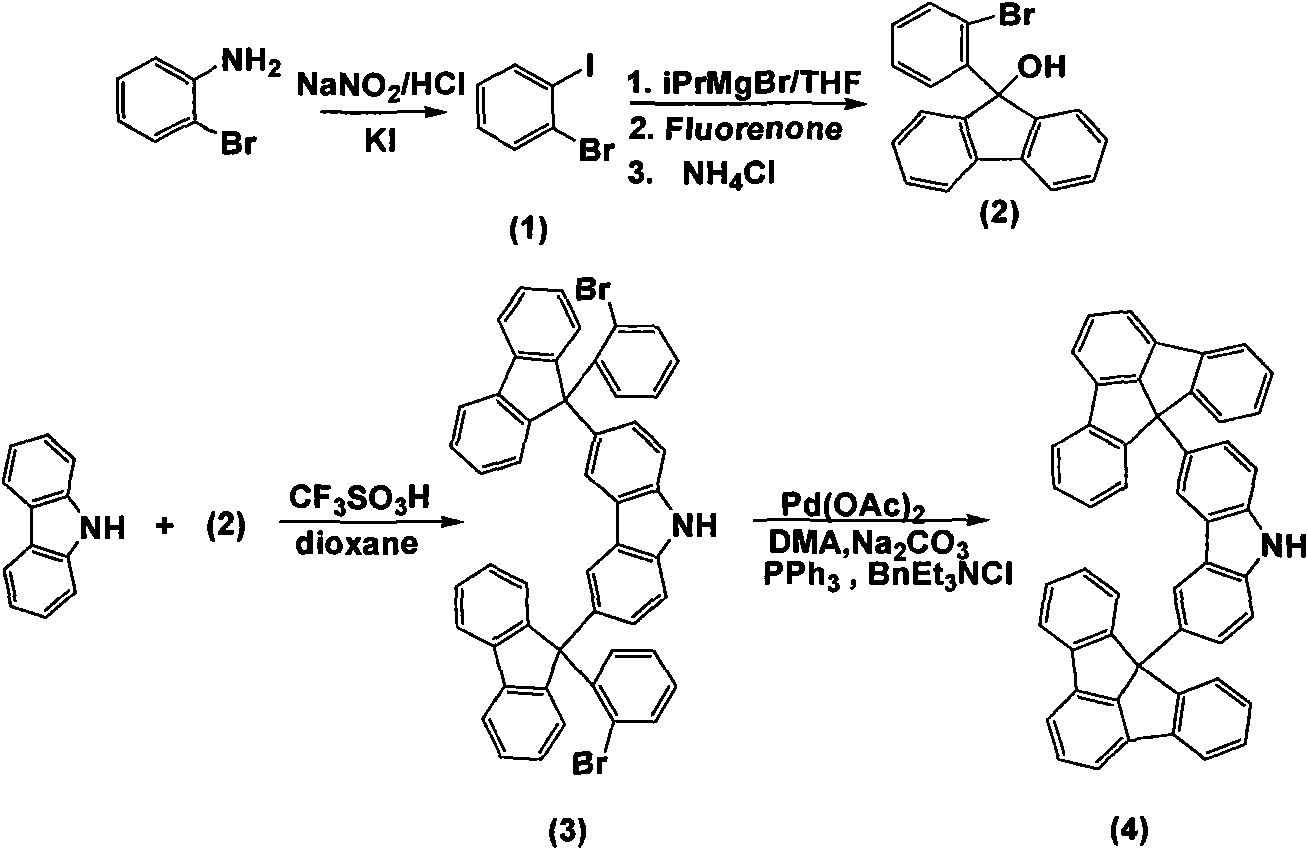
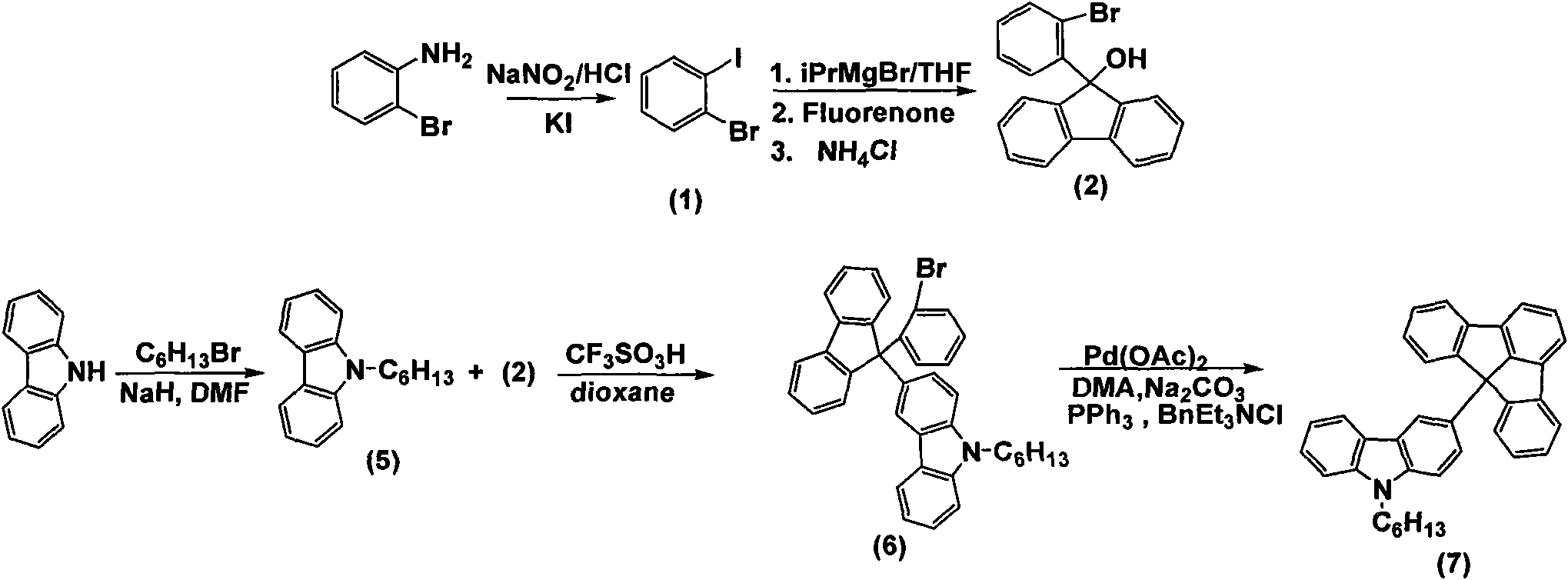
Fluoradene derivative and preparation method thereof
InactiveCN102030701ASpecific LuminescenceImprove thermal stabilityAmino preparation from aminesLuminescent compositionsHydrogen halideCarbazole
The invention discloses a fluoradene derivative and a preparation method thereof. The preparation method comprises the following steps of: introducing fluoradene rings into compounds, namely carbazole, hexyl carbazole, triphenylamine and hexyl triindole; and catalyzing intramolecular carbon-hydrogen bond activation and hydrogen halide elimination reaction through palladium and efficiently constructing a carbon ring or a heterocyclic compound, particularly constructing the carbon ring with a novel structure. The invention aims to synthesize and characterize a fluoradene structural unit-containing large-tension cyclic compound; and the novel fluoradene structural unit-containing compound is expected to be used in the field of organic photoelectric materials.
Owner:EAST CHINA NORMAL UNIV
Phenanthroimidazole-isoquinoline, derivative, preparation method and application thereof
ActiveCN103755702AReaction raw materials are readily availableReduce the amount addedOrganic chemistryFluorescence/phosphorescenceFluorescenceAlkyne
The invention discloses a phenanthroimidazole-isoquinoline, a derivative, a preparation method and the application thereof. The structural formulas of the phenanthroimidazole-isoquinoline and the derivative thereof are shown as a formula I. The preparation method comprises the following steps: taking 2-aryl-phenanthroimidazole shown in a formula II and internal alkynes shown in a formula III as oligomers, performing carbon-hydrogen bond activation and cyclization reactions under certain solvents and a temperature under the existence of trivalent rhodium or bivalent ruthenium catalysts and oxidizing agents, and obtaining the phenanthroimidazole-isoquinoline when Ar is substituted phenyl or substituted benzene rings. The method is convenient, universal and economic. The formula I has wide application prospects in organic optoelectronic materials and the analysis field based on fluorescent detection and has selective fuorescent responsiveness especially for ferric irons. Therefore, the formula I can be used for ferric iron detection.
Owner:TSINGHUA UNIV
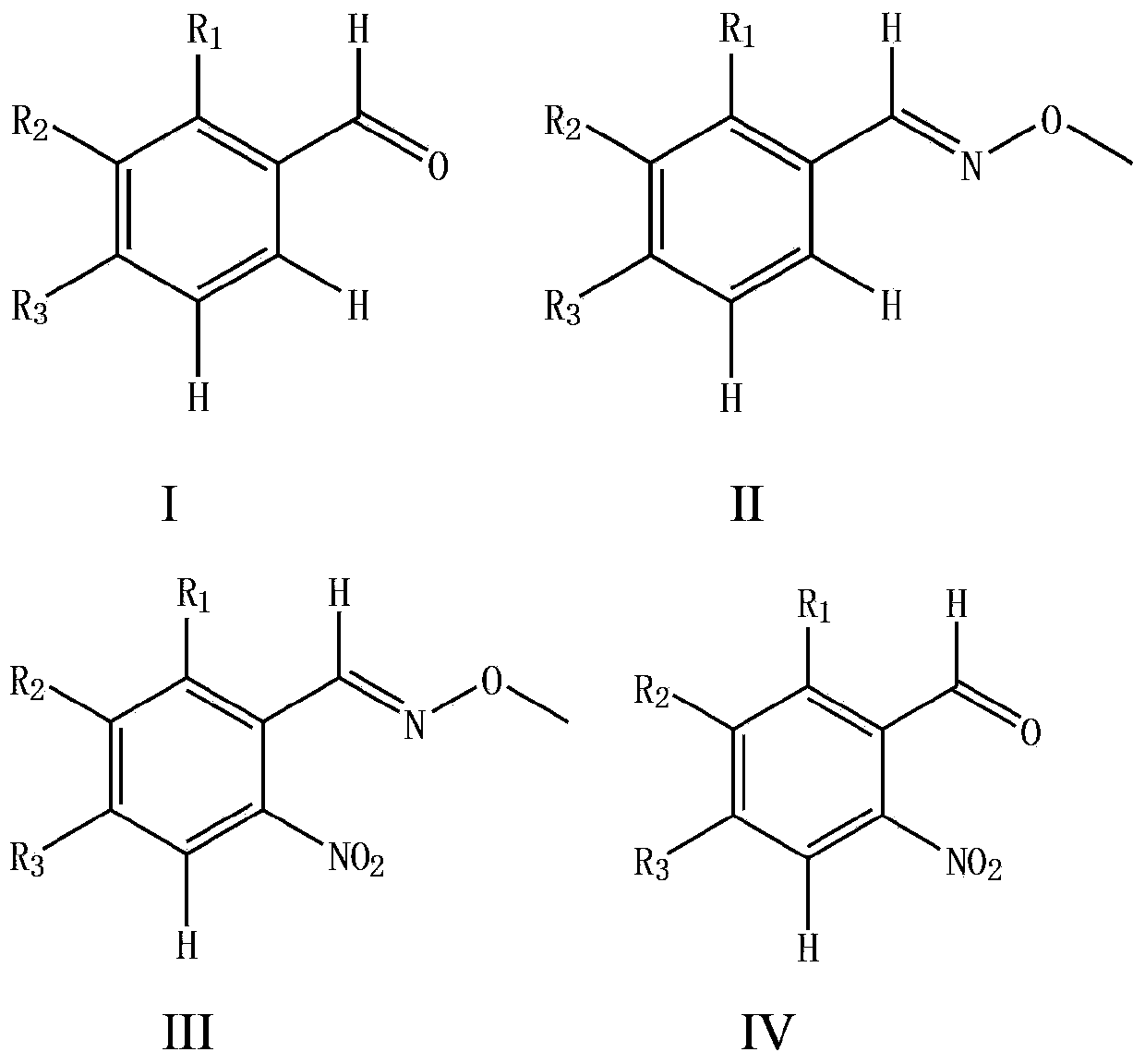
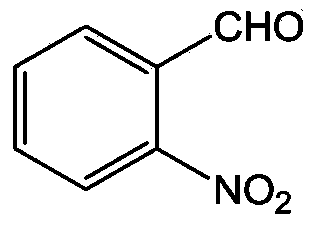
Synthesizing method for o-nitrobenzaldehyde compound
ActiveCN103467300AHigh yieldLow priceOrganic chemistryOrganic compound preparationOrganic acidBenzene
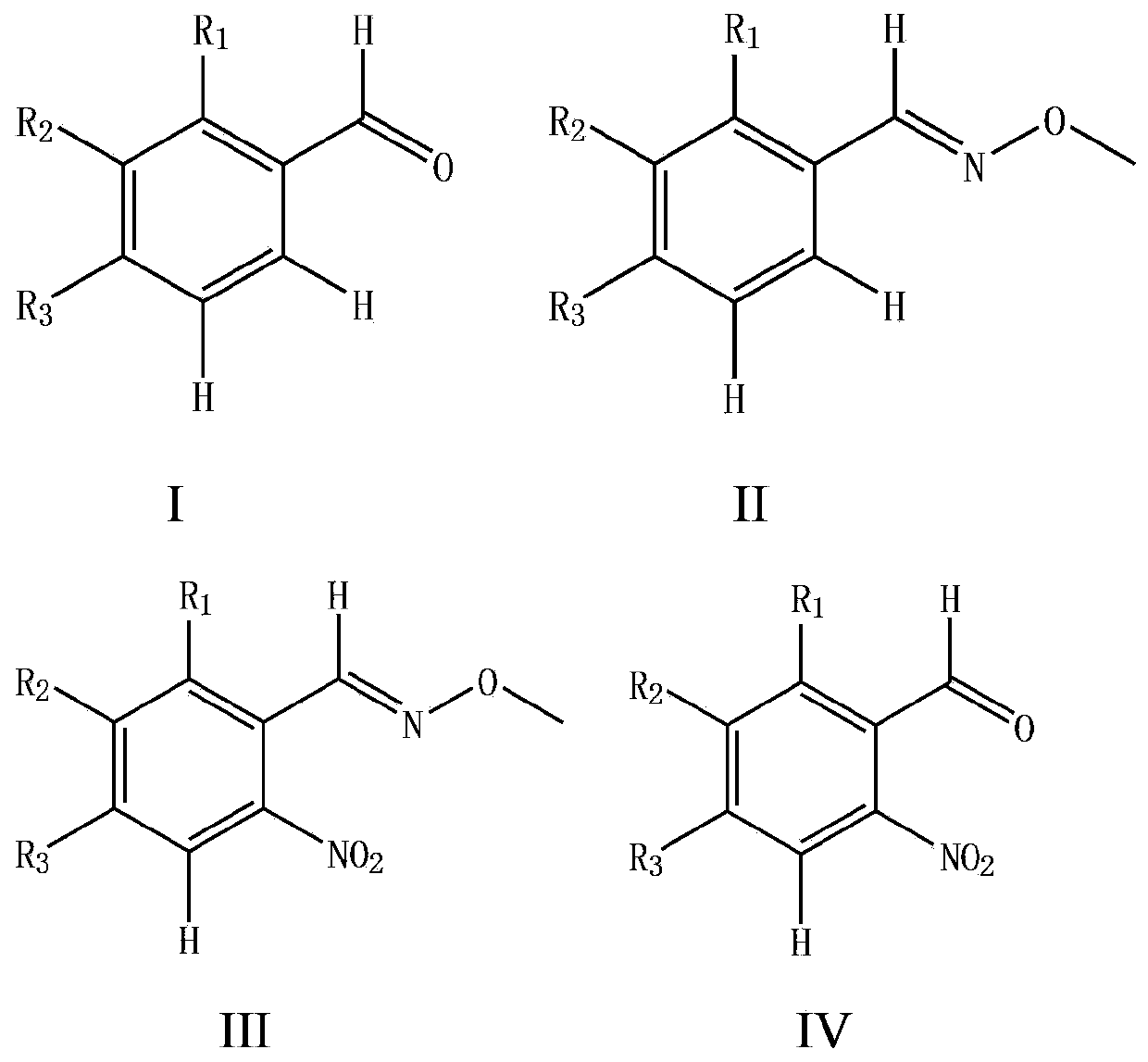
The invention provides a preparation method for an o-nitrobenzaldehyde compound. The method directly taking benzaldehyde compounds as starting raw materials comprises the following steps: firstly, converting a formyl group into an O-methyl oximido; secondly, taking divalent palladium salt as a catalyst, and realizing the carbon-hydrogen bond activation single nitration reaction on an o-position of an oximido under the condition that both an oxidant and a nitration agent exist; finally, removing the O-methyl oximido by using strong organic acid to obtain the o-nitrobenzaldehyde compound. The nitration method provided by the invention has the advantage of specificity in the o-position of a nitration position, the reaction process is safe and environment-friendly, the substrate is excellent in adaptability, and various substituents can realize o-position nitration; various benzaldehyde is directly taken as raw materials, so that the reaction steps are simple, and the synthesizing method is a novel route for synthesizing various o-nitrobenzaldehyde compounds containing substituents.
Owner:徐州宏阳新材料科技股份有限公司
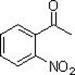
Method for synthesizing o-nitroacetophenone compound
InactiveCN101985424AGood substrate adaptabilityOrtho nitrificationOrganic chemistryOrganic compound preparationOrtho positionNitration
The invention provides a method for synthesizing o-nitroacetophenone compound, relating to a method for synthesizing organic compound. The invention takes acetophenone (can contain various substituent groups) as raw material directly, carbonyl group is converted into hydroxyimino, then palladium is taken as catalyst, carbon-hydrogen bond activation single nitration on the adjacent position of hydroxyimino is realized in the presence of oxidant and nitration agent, and finally hydroxyimino is hydrolyzed into corresponding ketone again under the action of acid, thus realizing nitration of adjacent position of carbonyl group of acetophenone and obtaining a series of o-nitroacetophenone compounds. The invention is safe and environmentally friendly, no waste gas or waste water is produced; adaptability of substrate is good, adjacent position nitration on various substituent groups can be realized; regioselectivity of nitration reaction is good; various nitroacetophenones are directly takenas raw materials, and reaction process is simple, thus being a new approach for synthesizing o-nitroacetophenone compound containing substituent groups.
Owner:ZHEJIANG UNIV OF TECH
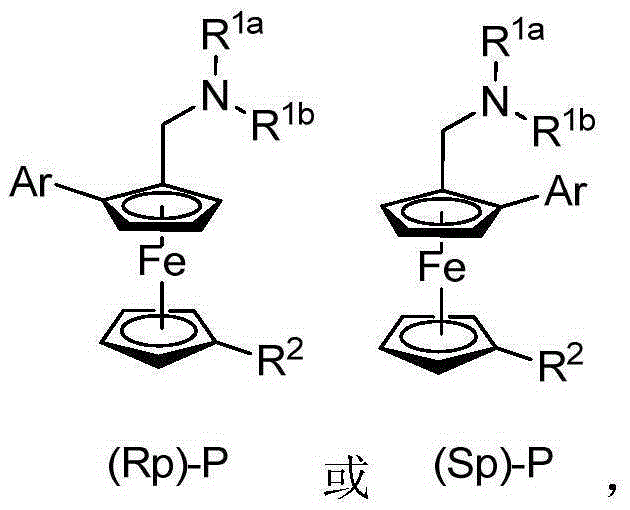
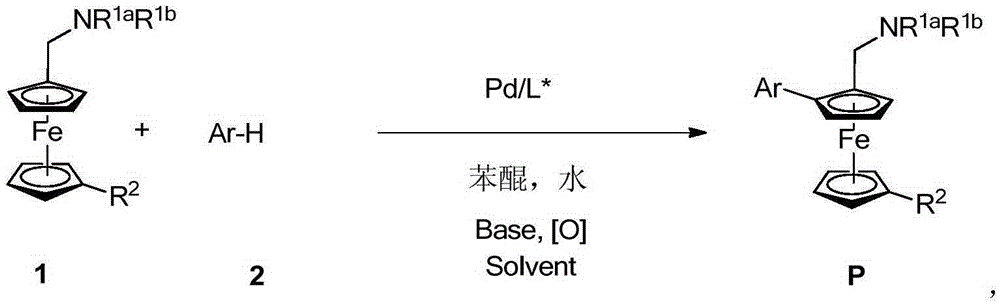
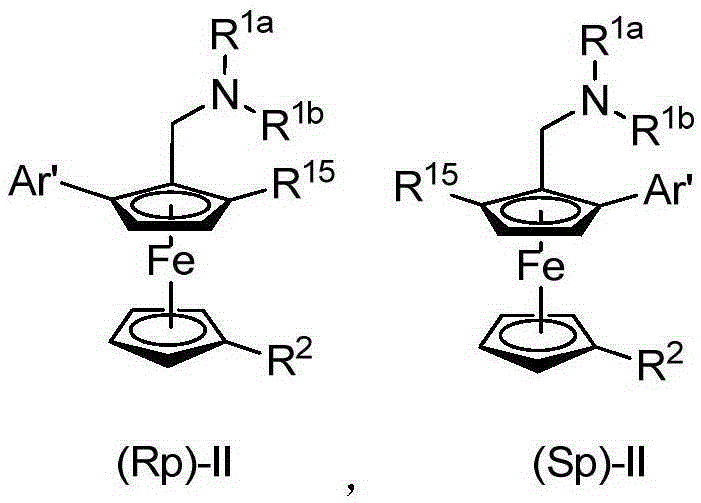
Planar chiral ferrocene compound, synthetic method and application
ActiveCN105254682ASimple ingredientsEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFerroceneStructural formula
The invention relates to a planar chiral ferrocene compound, a synthetic method and application. The planar chiral ferrocene compound has the structural formula shown as follows. Amine methyl substitutional ferrocene and heterocyclic aromatics are used as raw materials, chiral amino acid and palladium are used as catalysts, and the planar chiral ferrocene compound is synthesized with high efficiency and high enantioselectivity through double carbon-hydrogen bond activation. The planar chiral ferrocene compound can be conveniently converted into chiral ligand to be applied in a metal catalysis asymmetric reaction.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
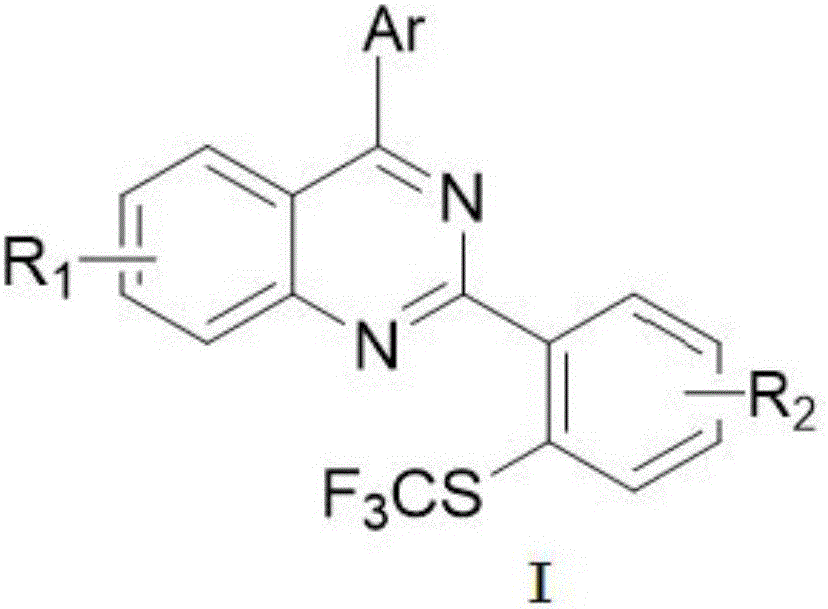
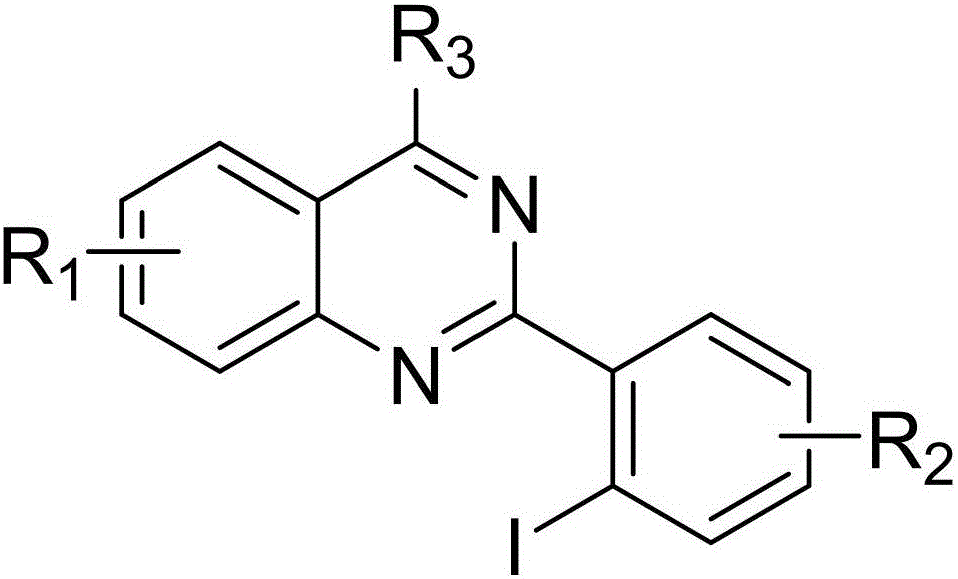
Method for preparing 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline
InactiveCN106632087AHigh yieldHigh purityOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsPentamethylcyclopentadieneTrifluoromethylation
The invention discloses a 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline compound and a preparation method thereof. The preparation method comprises the following steps: taking 2,4-diarylquinazoline as a reaction substrate, carrying out a reaction with NIS under the catalytic action of pentamethylcyclopentadienylrhodium (III) chloride dimer / silver hexafluoroantimonate under a condition of 80 DEG C, carrying out a reaction with a sulfur trifluoromethylation reagent, and taking copper iodide as a catalyst, wherein the reaction temperatire is 85 DEG C, and the reaction time is 7-10 hours; performing carbon hydrogen bond activation process, thereby obtaining the 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline compound. The preparation method disclosed by the invention is mild in reaction conditions, easy and convenient to operate, low in cost, few in side reactions, high in product purity and convenient for separation and purification and can be suitable for large-scale preparation, and the obtained product has high drug activity and excellent potential application prospects.
Owner:JIANGXI NORMAL UNIV
Hydrogen production catalyst system, hydrogen production system comprising same and applications of hydrogen production catalyst system
ActiveCN107128875AImprove hydrogen production efficiencyRaw materials are cheap and easy to getGas treatmentMolecular sieve catalystsNano catalystChemical industry
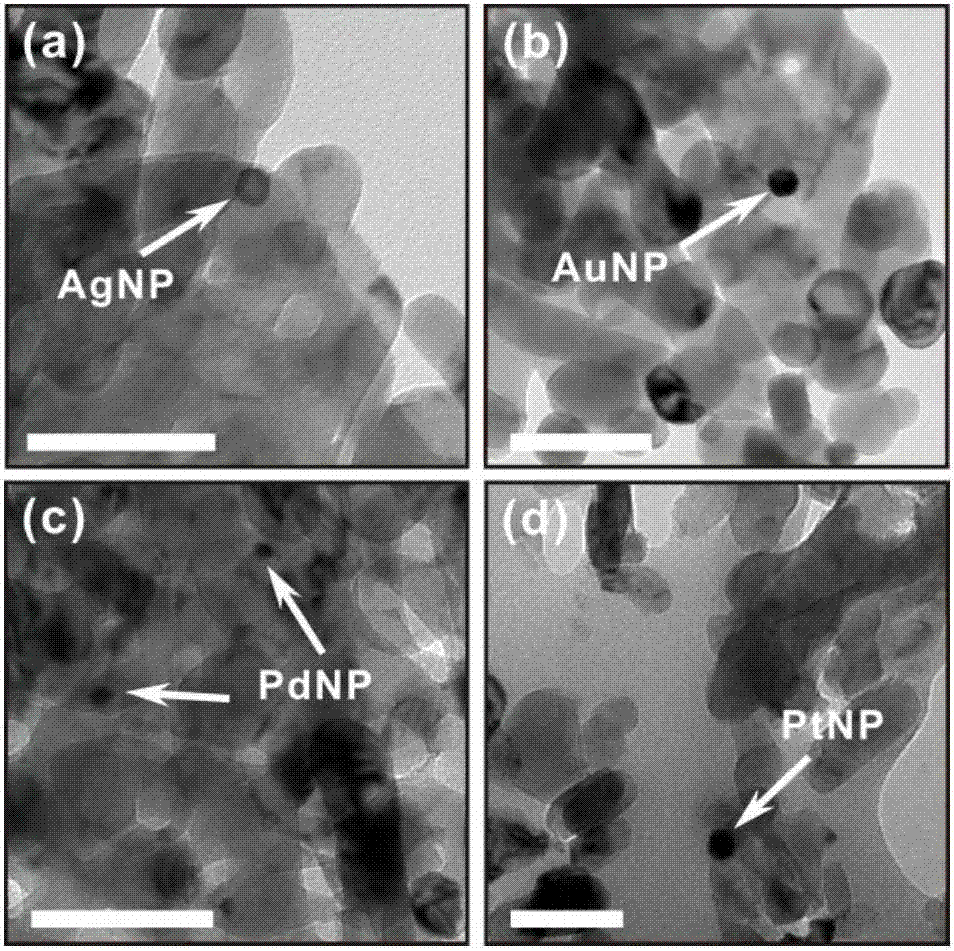
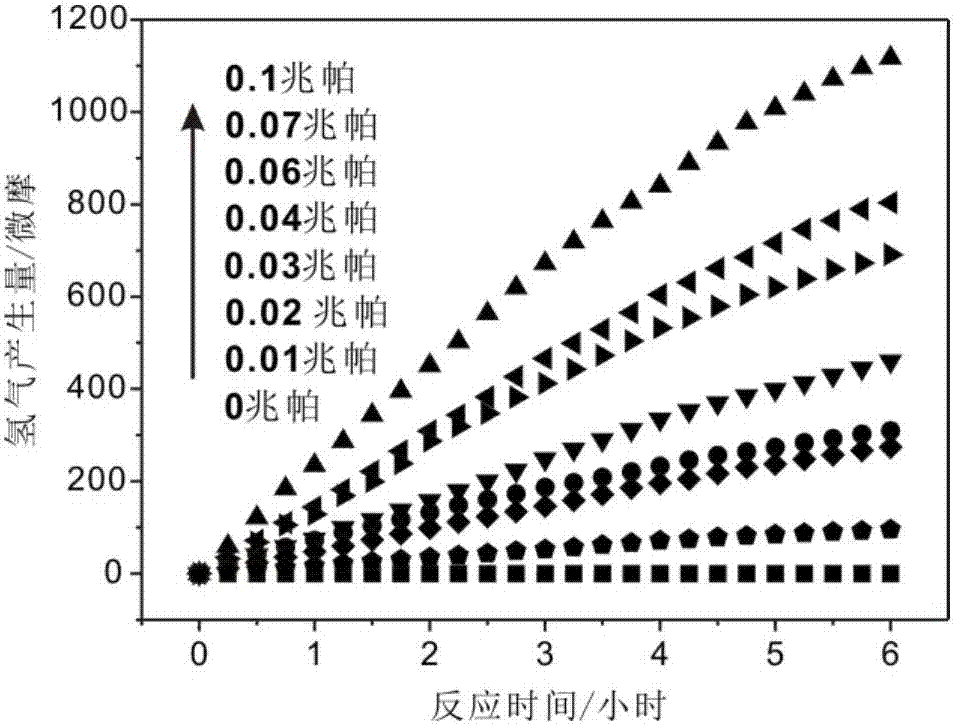
The invention provides a hydrogen production catalyst system, a hydrogen production system comprising the same and applications of the hydrogen production catalyst system. The hydrogen production catalyst system comprises a metal nano catalyst and a biomass water solution. The metal nano catalyst catalyzes the biomass water solution to produce hydrogen. The hydrogen production efficiency and hydrogen production capacity of the catalyst system are controlled by the amount of oxygen. The hydrogen production efficiency of the catalyst system is increased, when the pressure or concentration of oxygen in the hydrogen production reaction system increases. For the first time, oxygen molecules are used to control the production speed of hydrogen. Moreover, the hydrogen production method is efficient, clean, and environmentally friendly, and is easy to control; and the equipment is simple. At a low temperature (not lower than 0 DEG C), water hydrolysis and biomass hydrolysis can be realized at the same time, the energy consumption is greatly reduced, an energy-consumption-free reaction condition can be almost realized, and the catalytic method is sustainable and has a wide application prospect. At the same time, the catalyst system can be applied to fields such as hydrogen production, energy and chemical industry, battery, water treatment, paint, functional textile, indoor air purification and formaldehyde removing, petrochemical engineering, carbon-hydrogen bond activation, pharmacy, hydrogen-containing water preparation, and the like.
Owner:ZHEJIANG SCI-TECH UNIV
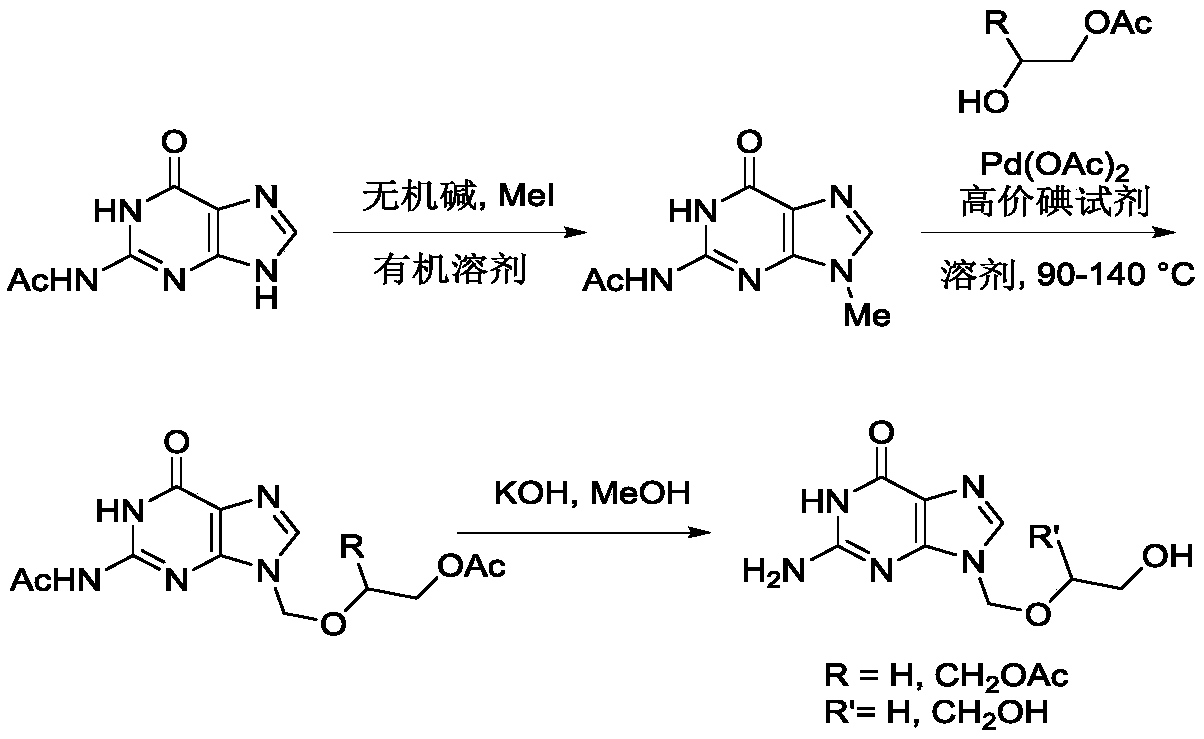
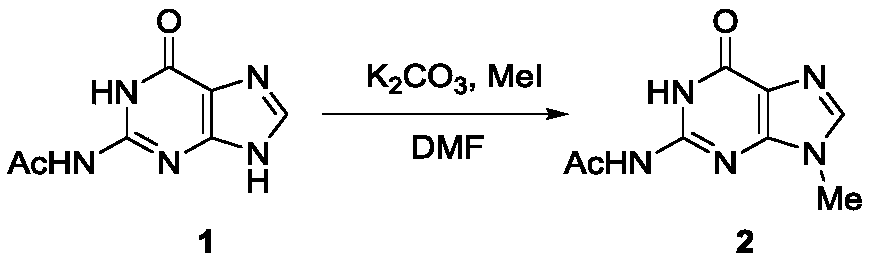
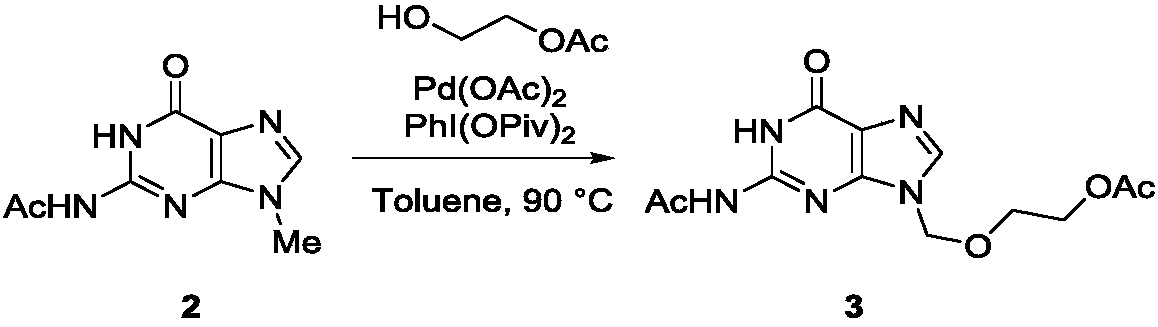
Method for synthesizing acyclovir and ganciclovir by carbon-hydrogen bond activation
The invention discloses a method for synthesizing acyclovir and ganciclovir by carbon-hydrogen bond activation and belongs to the field of organic synthesis. The method comprises that inexpensive guanine as a raw material undergoes methyl protection on 9th NH, a high-valent iodine reagent and monoacetyl-protected ethylene glycol or 1, 2-isopropylidene-protected glycerol are added into the raw material under catalysis of palladium acetate, the mixture undergo a heating reaction to produce acetyl-protected acyclovir or acetyl-protected ganciclovir, and the acetyl group is removed by an inorganicalkali alcohol solution so that acyclovir and ganciclovir are obtained. The method utilizes cheap and easily available raw materials, prevents use risk and corrosive reagents, has the advantages of short reaction route, simple operation, high atomic economy and high total product yield, provides a novel synthesis route of acyclovir and ganciclovir and has a potential application prospect.
Owner:XINYANG NORMAL UNIVERSITY
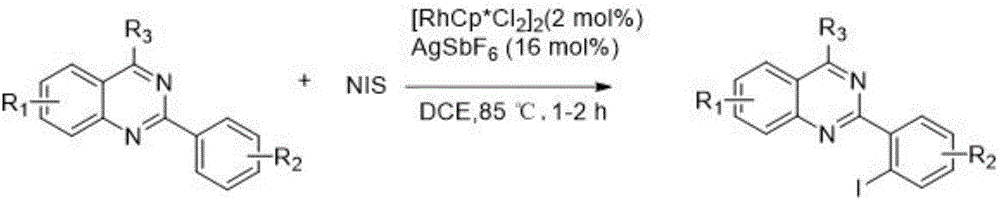
2-(2-iodoaryl)quinazoline compound and preparation method thereof
ActiveCN106632086AImprove efficiencyEasy to operateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPentamethylcyclopentadieneReaction temperature
The invention discloses a 2-(2-iodoaryl)quinazoline compound and a preparation method thereof. The 2-(2-iodoaryl)quinazoline compound is prepared by using 2-arylquinazoline as a reaction substrate through the process of carbon-hydrogen bond activation iodination with iodosuccinimide under the catalytic action of dichloro(pentamethylcyclopetadienyl)rhodium (III) dimer / silver hexafluoroantimonate, wherein the reaction temperature is 85 DEG C, and the reaction time is 1 to 2 hours. The preparation method provided by the invention has the advantages of high yield, excellent reaction chemical selectivity, mild reaction conditions, simple and convenient operation, low cost, less side reaction and high product purity; and the product disclosed by the invention is a novel compound, facilitates to separation and purification, is applicable to large-scale preparation, and has good application value and potential biological activity.
Owner:JIANGXI ACAD OF FORESTRY
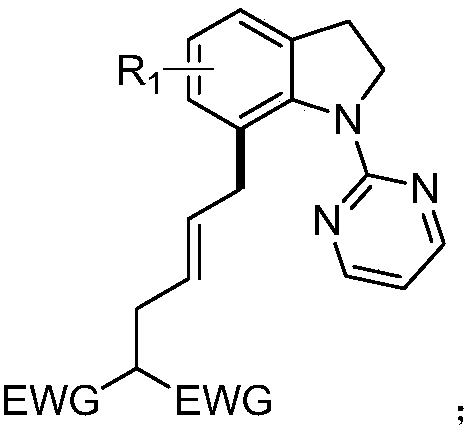
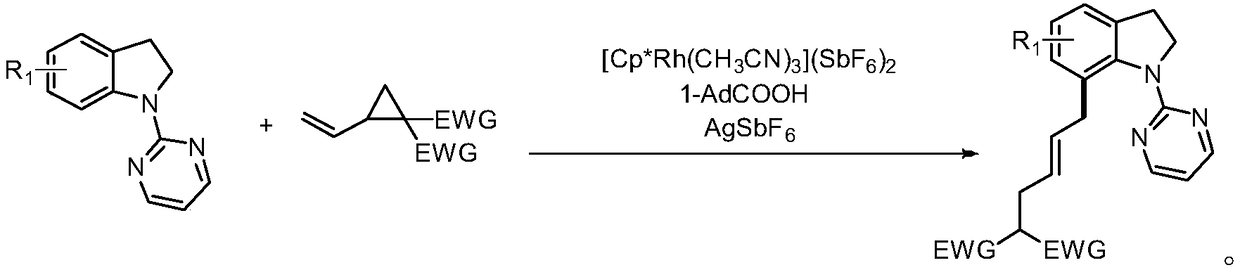
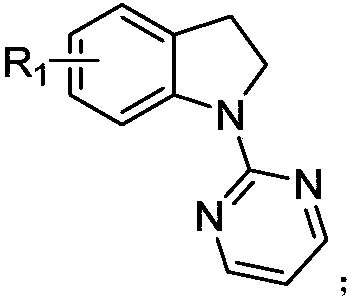
7-alkyl-N-pyrimidylindoline compound and synthesis method thereof
ActiveCN109180648ARich types of functionalization reactionsMultiple Feasibility PathwaysOrganic chemistryChromatographic separationCarbon–carbon bond
The invention discloses a 7-alkyl-N-pyrimidylindoline compound and a synthesis method thereof. The 7-alkyl-N-pyrimidylindoline compound has a general structural formula shown in the description. The synthesis method comprises: adding an N-pyrimidylindoline compound and a vinyl cyclopropane compound into a reaction tube in an argon environment, adding 1-adamantanecarboxylic acid, [Cp*Rh(CH3CN)3](SbF6)2 and silver hexafluoroantimonate into the reaction tube, then adding methanol into the reaction tube, carrying out a reaction process at 80-120 DEG C for 12h, and then carrying out leaching, thinlayer chromatographic separation and drying to obtain a desired product. The metal ruthenium (III) is used as a catalyst and methanol is used as a reaction solvent so that the carbon-carbon bond cleavage of vinylcyclopropane and activation of a 7-site carbon-hydrogen bond of an indoline compound are realized. The long alkyl chain with double bonds realizes a further functionalization reaction. Thesynthesis method is simple and efficient and has good selectivity. The 7-alkyl-N-pyrimidylindoline compound is easy to purify.
Owner:ZHENGZHOU UNIV
Carbon-hydrogen bond activation method for non-metal participated inert alkane
ActiveCN112062707AMild reaction conditionsNo heating requiredOrganic chemistryChemical recyclingAlkaneDistillation
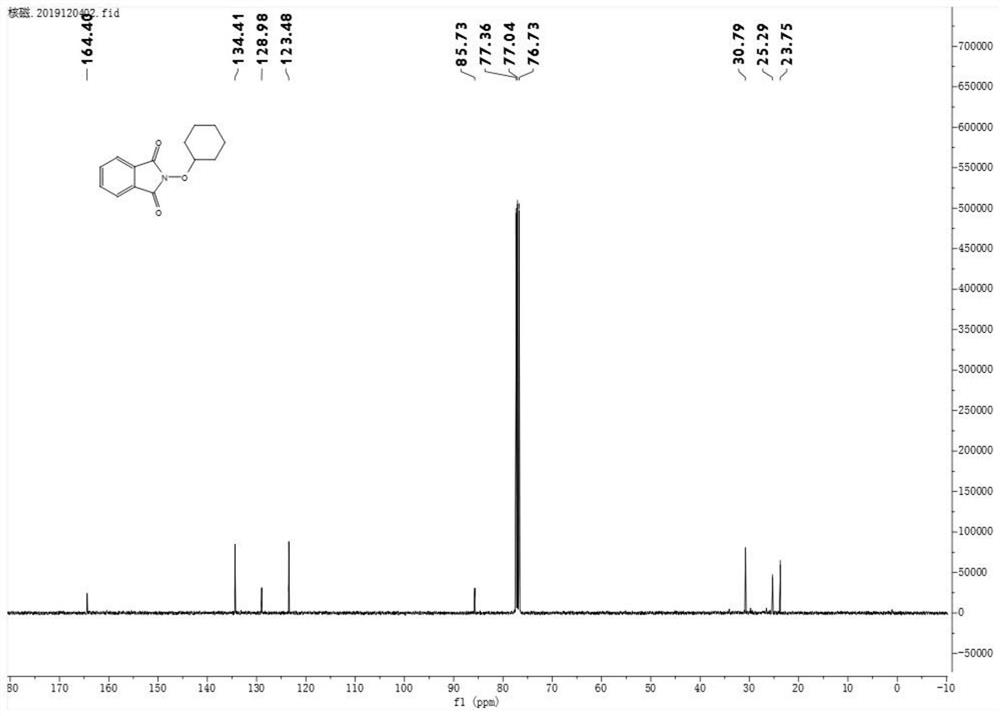
The invention discloses an activation method of a non-metal catalyzed inert alkane carbon-hydrogen bond. The activation method comprises the following steps: S1, dissolving a compound inert alkane andN-hydroxyphthalimide into dichloromethane; S2, adding iodobenzene acetate into the system; S3, after N-hydroxyphthalimide disappears, carrying out reduced pressure distillation on the reaction systemto remove the low-boiling-point organic solvent; and S4, carrying out silica gel column chromatography separation to obtain the compound I. The method disclosed by the invention comprises the step ofadding the inert alkane compound into a solvent system of iodobenzene acetate and Nhydroxyphthalimide, so that the reaction condition is mild, heating is not needed, no metal participates, the methodis simple and efficient, the post-treatment is simple, inert gas or oxygen conditions are not needed, and the development concept of green chemistry is met.
Owner:FUYANG NORMAL UNIVERSITY
Method for preparing sulfonyl fluoride compound by activating carbon-hydrogen bond
InactiveCN108484454AWide range of usesImprove toleranceSulfonic acid preparationBiological activationSolvent
The invention discloses a method for preparing a sulfonyl fluoride compound by activating a carbon-hydrogen bond. The method comprises the following steps: mixing a raw material Weinreb amide with ethylenesulfonyl fluoride (ESF), a ruthenium catalyst, an additive, an oxidant and a solvent, performing a reaction at 20-120 DEG C for 15 h or more, and separating and purifying the obtained reaction product to obtain the sulfonyl fluoride compound. Compared with existing methods, the method has the characteristics of simplicity, high efficiency and good functional group tolerance. The method for preparing 2-aryl-ethylenesulfonyl fluoride, a sulfonyl fluoride-substituted lactone compound and a sulfonyl fluoride-substituted gamma-lactam compound through realizing an oxidative coupling reaction ofan N-methoxy-N-methyl amide compound and ethylenesulfonyl fluoride by ruthenium-catalyzed direct carbon-hydrogen bond functional group activation.
Owner:WUHAN UNIV OF TECH
Preparation method of 4-aryl-2-(2-(thiotrifluoromethyl)aryl)quinazoline
ActiveCN108976173AHigh yieldMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsPentamethylcyclopentadieneIodide
The invention discloses a 4-aryl-2-(2-(thiotrifluoromethyl)aryl)quinazoline compound and a preparation method thereof. 2,4-diarylquinazoline is used as a reaction substrate to react with NIS under thecatalytic effect of dichloro(pentamethylcyclopentadienyl) rhodium(III) dipolymer / silver hexafluoroantimonate and then react with a thiotrifluoromethy reagent for 7 to 10 hours at the reaction temperature of 85 DEG C by adopting cuprous iodide as a catalyst by virtue of a carbon-hydrogen-bond activation process, so that the 4-aryl-2-(2-(thiotrifluoromethyl)aryl)quinazoline compound is obtained. The preparation method of the invention is mild in reaction condition, simple in operation, low in cost, less in side reaction, high in product purify, convenient in separation purification and suitablefor mass preparation, and the prepared product is good in drug activity and good in potential application prospect.
Owner:JIANGXI NORMAL UNIV
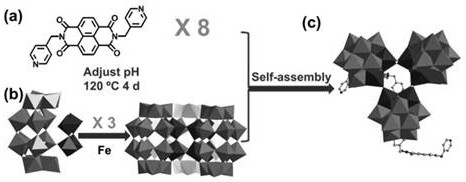
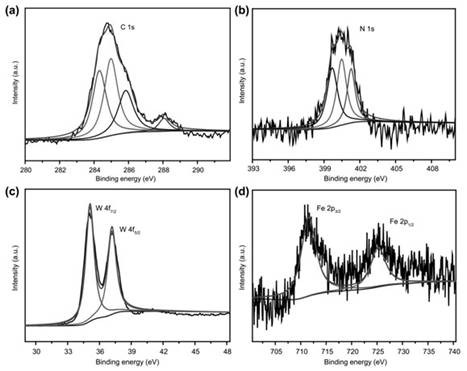
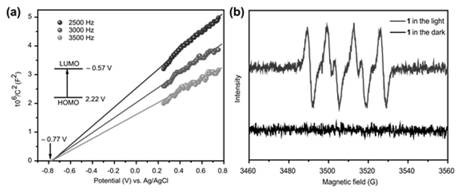
Polyacid-based organic-inorganic complex, preparation method thereof, and application of the polyacid-based organic-inorganic complex as photocatalyst in selective oxidation of toluene into benzaldehyde
ActiveCN111644205APromote productionGuaranteed efficient selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystBenzaldehyde
The invention discloses a polyacid-based organic-inorganic complex. The chemical formula of the polyacid-based organic-inorganic complex is K4(H2O)8H26(C26H16N4O4)8[P6W48Fe6O180].7H2O. The complex generates photo-induced electrons and holes under the illumination condition, the holes promote formation of benzyl free radicals, the electrons reduce oxygen into superoxide free radicals to promote formation of benzaldehyde, and efficient selectivity of catalytic reaction is achieved. The catalytic process is heterogeneous, the catalyst can be recovered through centrifugal drying, cyclic utilization is realized, the catalytic efficiency is not obviously reduced, and the reaction still keeps very high selectivity. The polyacid-based organic-inorganic complex material provides a scientific basisfor the development of fine chemical engineering in China in the aspect of carbon-hydrogen bond activation.
Owner:HENAN UNIVERSITY
Diaryl-azepin-5-one compound synthesis method
InactiveCN104910073AReduce pollutionEasy to operateOrganic chemistryPtru catalystPharmaceutical Substances
The invention relates to a diaryl-azepin-5-one compound synthesis method. The method comprises that o-haloarylmethanol and aryl cyanide (or a mixture of aryl methanol and o-haloarylmethanol), a ruthenium catalyst, a palladium salt, azacycloimidazolium salt and an alkali are added into an organic solvent and the solution is heated in a N2 protective atmosphere so that through a hydrogen transfer reaction and a carbon-hydrogen bond activation reaction, the diaryl-azepin-5-one compound is produced. The method provides an economic and practical preparation method for synthesis of the substituted diaryl-azepin-5-one derivative with drug activity. The method has simple processes, utilizes cheap reaction substrates with a wide source scope, produces small environment pollution, has high reaction economy and efficiency and has an important application value.
Owner:LUOYANG NORMAL UNIV
Ruthenium catalytic 3-substituted isocoumarin synthesis method
ActiveCN110357848ARealize the ring closing reactionEasy to operateOrganic chemistrySynthesis methodsRuthenium
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Method for preparing polysubstituted indole from aryl hydrazine and alkyne
The invention discloses a method for preparing polysubstituted indole. The method comprises the following step: carrying out alkyne cyclization reaction based on hydrocarbon chain activation on aryl hydrazine hydrochloride disclosed as Formula II, alkyne disclosed as Formula III, catalyst, alkali and additives to obtain the compound disclosed as Formula I. The accessible raw materials are subjected to on-pot reaction to generate orientating group in situ in the reaction without presetting the orientating group on the substrate. After the cyclization reaction, the orientating group is automatically removed, and the obtained NH indole can be conveniently derived to obtain the N-substituted indole and has favorable compatibility with multiple substituent groups; no additional oxidizer and strong acid or strong alkali are needed in the reaction; the atomic economical efficiency is high; and although the catalyst is not cheap, the addition amount is small.
Owner:TSINGHUA UNIV
Method for preparing tetrasubstituted furan compound based on carbon-hydrogen bond activation
ActiveCN108976186AStarting materials are cheap and readily availableReduce dosageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFuranOrganic synthesis
The invention provides a method for preparing a tetrasubstituted furan compound based on carbon-hydrogen bond activation, belongs to the field of organic synthesis, and relates to a method for synthesizing a tetrasubstituted furan compound through a [3+2] cycloaddition reaction of beta-ketoester and a propargyl compound by usiong a C-H bond activation strategy, wherein the used copper catalyst isgenerated in situ from a copper salt and a 1,10-azaphenanthrene ligand in various polar and non-polar solvents. According to the present invention, various tetrasubstituted furan compounds can be conveniently synthesized, the yield can be up to 94%, and the method has characteristics of simple operation, easily available raw materials, wide application range of substrate, high reaction activity and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing porous manganese oxide catalyst
ActiveCN108246285AImprove performanceEfficient activated oxidationPreparation by oxidation reactionsOrganic compound preparationLipid formationSolvent free
The invention relates to a preparation technology of a catalytic material, and aims to provide a method for preparing a porous manganese oxide catalyst. The method comprises the following steps: carrying out foaming treatment by using manganese salt, a hydrate of the manganese salt or an aqueous solution of the manganese salt as a precursor and a lipid polymer prepared from organic acid and alcohols in situ as a foaming agent, uniformly stirring after mixing the precursor, the organic acid, the alcohols used for carrying out esterification reaction with acid, the water and ethanol which is used as a solvent according to a mass ratio of 10 to 10 to 10 to 30 to 30, and heating and stirring under 50 to 170 DEG C until expanding and foaming; carrying out drying and roasting treatment on a thick colloidal-state matter obtained through foaming, thus obtaining the porous manganese oxide catalyst. The porous manganese oxide catalyst prepared by the invention has a porous structure and has excellent performance aiming at C-H bond activation reaction. Even under a solvent-free condition, the porous manganese oxide catalyst prepared by the invention is still capable of realizing efficient activated oxidation on ethylbenzene C-H bonds.
Owner:ZHEJIANG UNIV
Method for synthesizing azaindoline derivative
ActiveCN110483509AImprove universalityAtom economy is highOrganic chemistryOrganic synthesisNitrogen
The invention discloses a method for synthesizing azaindoline derivatives, and belongs to the technical field of organic synthesis. Pyridine carbon-hydrogen bond activation functionalization is catalyzed by rare earth, so that synthesis of azaindoline derivatives with diversified structures is achieved. Specifically, in a rare earth catalytic system, various alkenyl-substituted aminopyridine is used as a raw material to prepare the azaindoline derivative. The method disclosed by the invention is wide in raw material source or easy to prepare, simple and convenient to operate, controllable in selectivity, high in yield, mild in condition and wide in universality.
Owner:WENZHOU UNIVERSITY
Method for synthesizing olefin through selective desaturation of inert carbon-carbon bonds
ActiveCN113173862AHigh stereoselectivityEasy to retouchOrganic compound preparationCarboxylic acid amides preparationPtru catalystDouble bond
The invention relates to a method for synthesizing olefin by selective desaturation of inert carbon-carbon bonds. The method comprises the following steps of: synthesizing a chlorinated amide compound serving as a raw material in the presence of visible light, a ruthenium catalyst and a ligand, and post-treating reaction liquid to obtain a remote alkenyl amide compound. In the presence of the ruthenium catalyst and the phenylpyridine ligand, intramolecular carbon-hydrogen bond activation of the amide compound is successfully realized, and the remote olefin compound is synthesized; according to the method, chloro-amide which is simple and easy to obtain is used as a raw material, the substrate application range is wide, and the reaction efficiency is high; in the free radical migration process, 1, 5 migration occurs all the time along with beta-H elimination, terminal alkene and conjugated double bonds are used as main products, and high regioselectivity is achieved; and the maximum E / Z value of the obtained olefin compound is greater than 20: 1, and the stereoselectivity is good. The double bond of the compound can be further converted into other functional groups, the conversion process is simple in one step, and the obtained derivative can be used as a medical intermediate and has high potential application value.
Owner:YANGZHOU UNIV
Method for highly enantioselective catalytic synthesis of chiral quinolinone compound
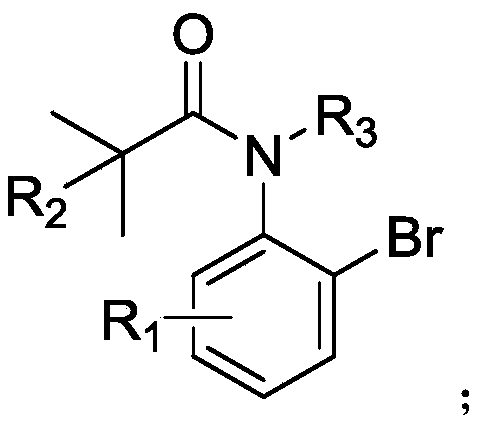
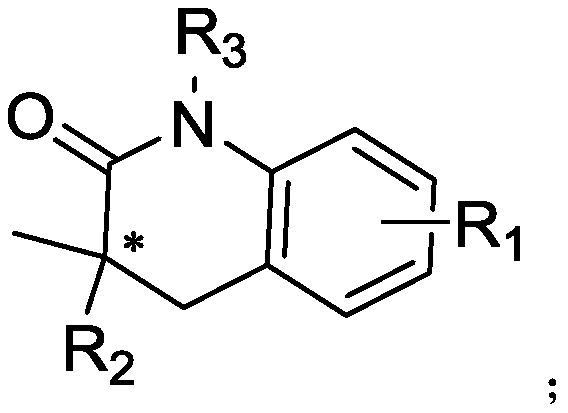
The present invention provides a method for highly enantioselective catalytic synthesis of a chiral quinolinone compound, particularly a method that adopts bromoarylamide compound as a starting raw material and that includes intramolecular asymmetric carbon-hydrogen bond activation of the amide compound under existence of a palladium catalyst and a chiral ligand to synthesize the chiral quinolinone compound. A non-optically active raw material is adopted, a substrate has a wide application range, and the reaction efficiency is high, and the obtained optically active quinolinone compound can beconverted into an alpha-amino amide compound having a quaternary carbon chiral center. The obtained chiral quinolinone compound can be used as a chiral fragment of quinolinone type drugs, or as an amide small molecule catalyst for catalyzing asymmetric reactions; the quinolinone structure can be further converted to obtain alpha-amino acids; and the chiral quinolinone compound can also be used asa food additive.
Owner:YANGZHOU UNIV
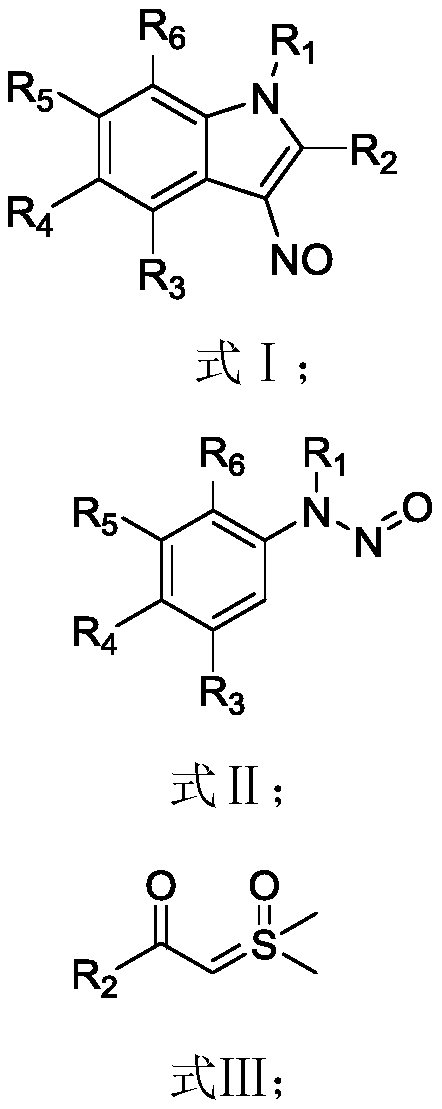
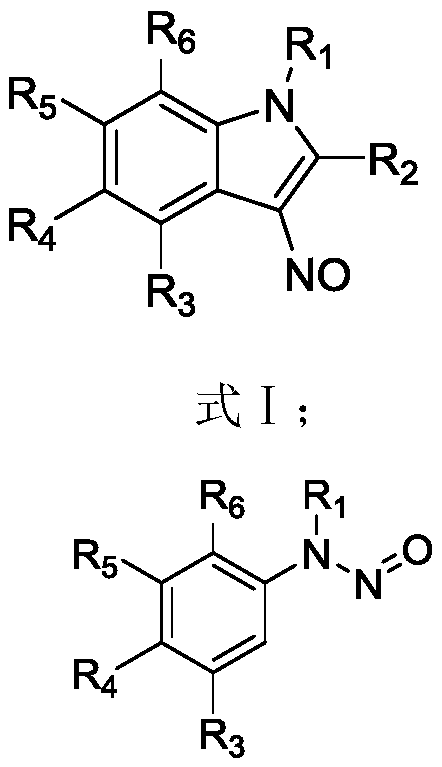
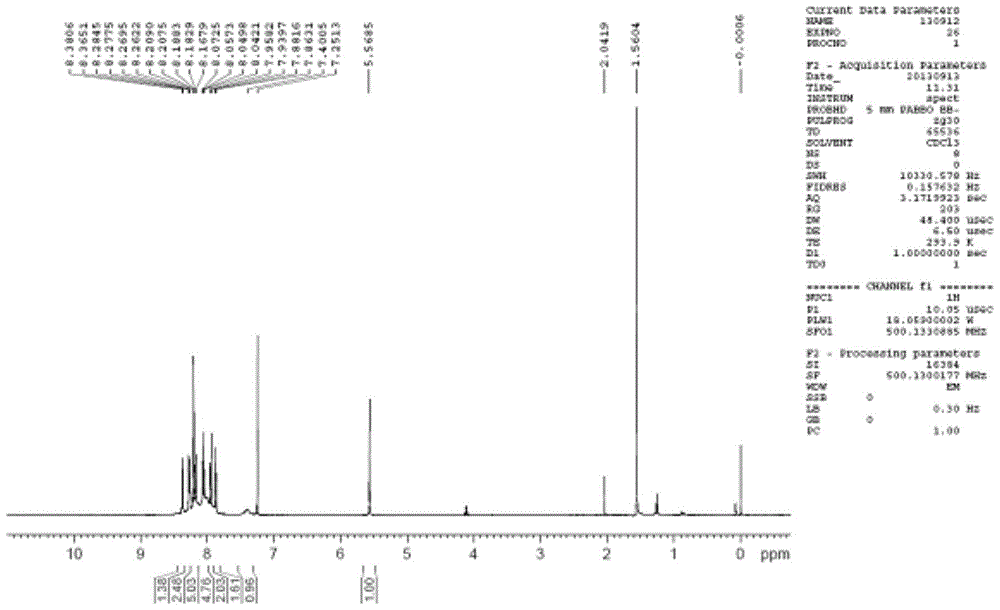
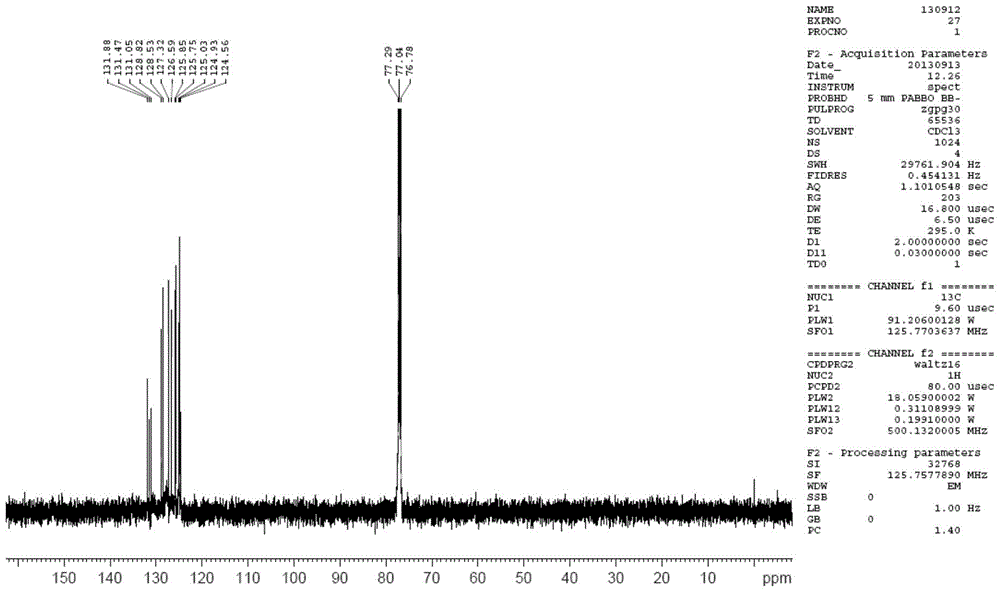
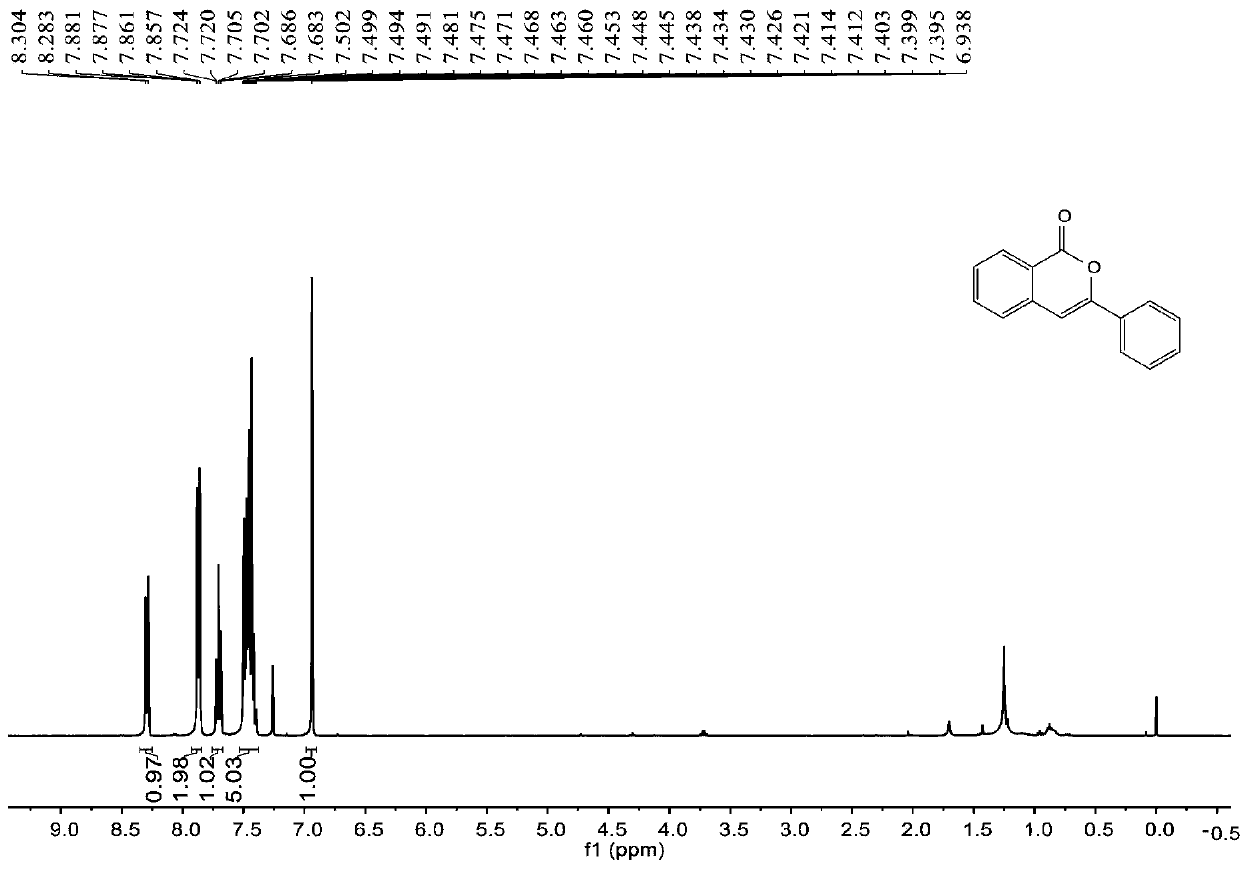
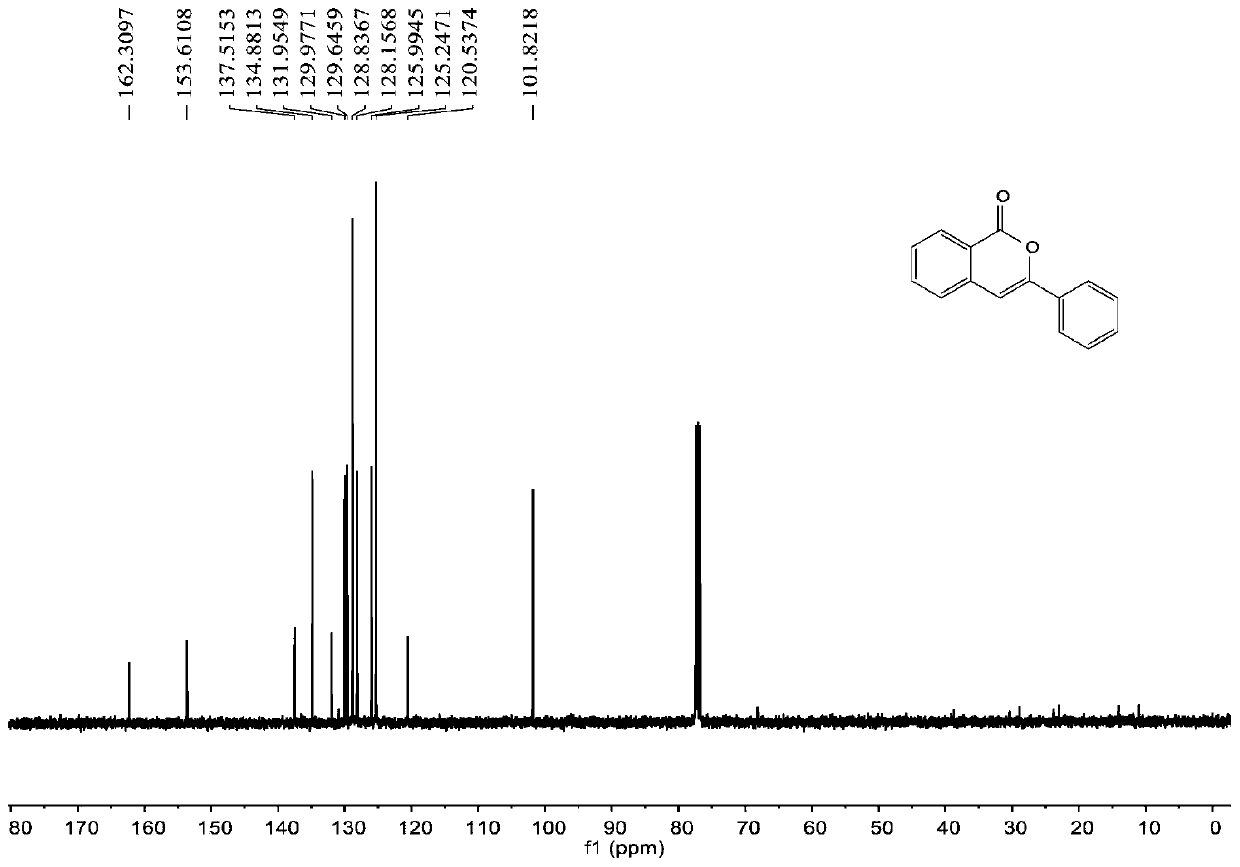
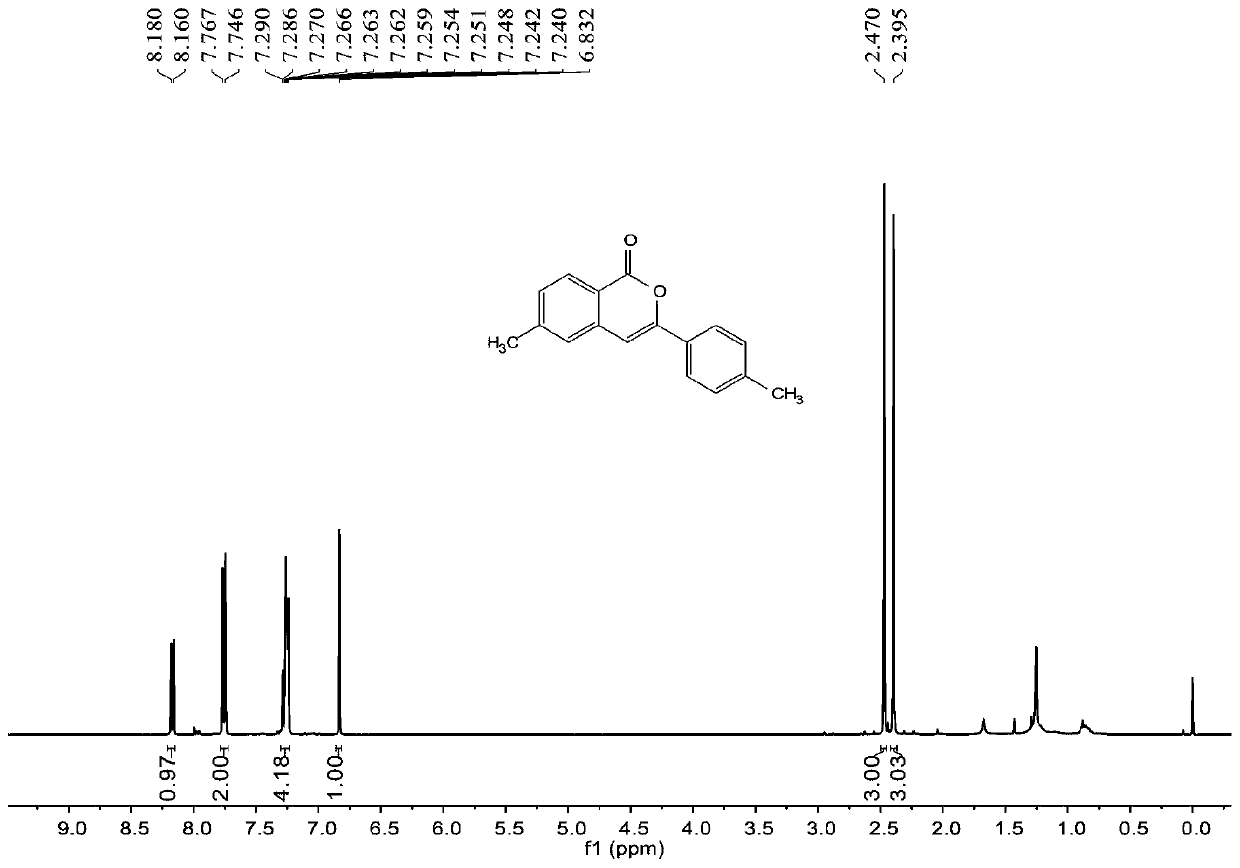
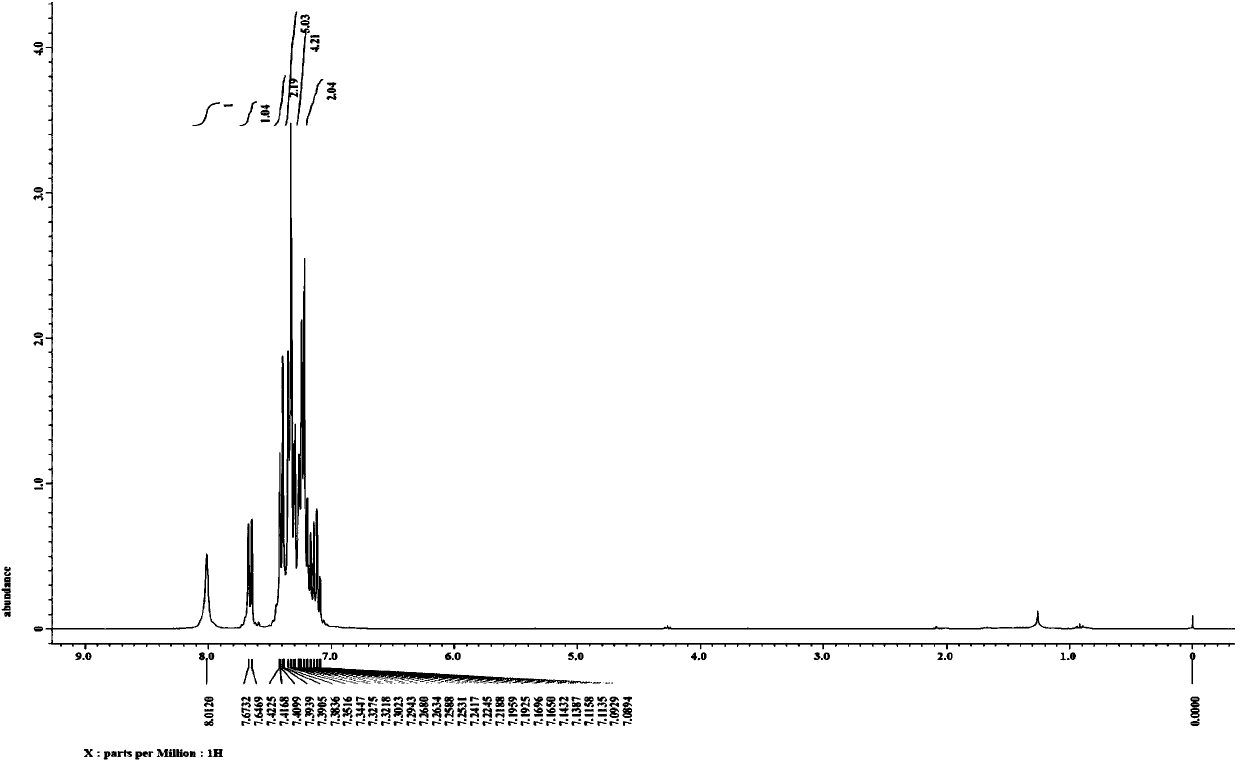
Synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds
ActiveCN113105460AImprove universalityRealize double carbon-hydrogen bond activation reactionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylPtru catalyst
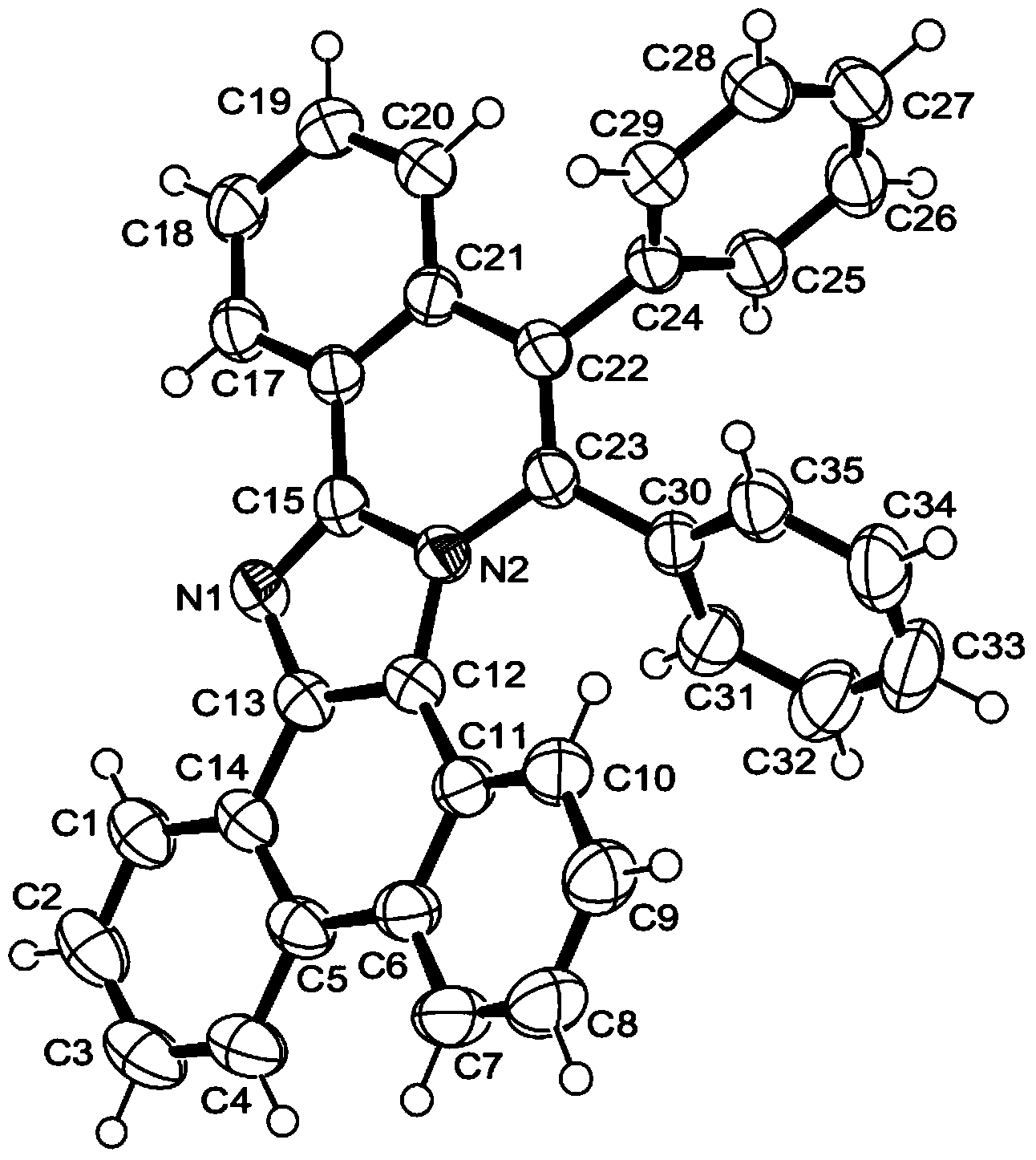
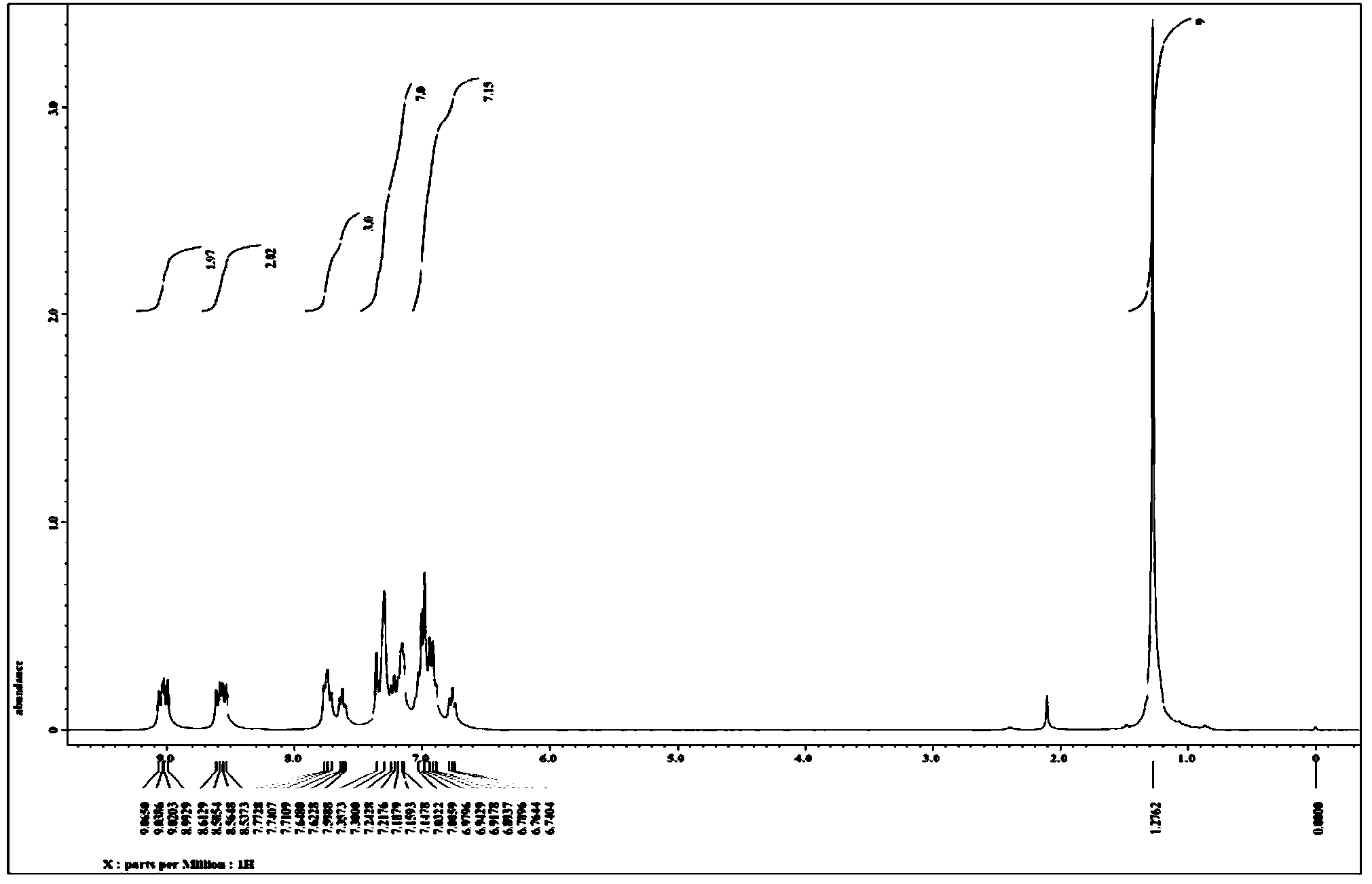
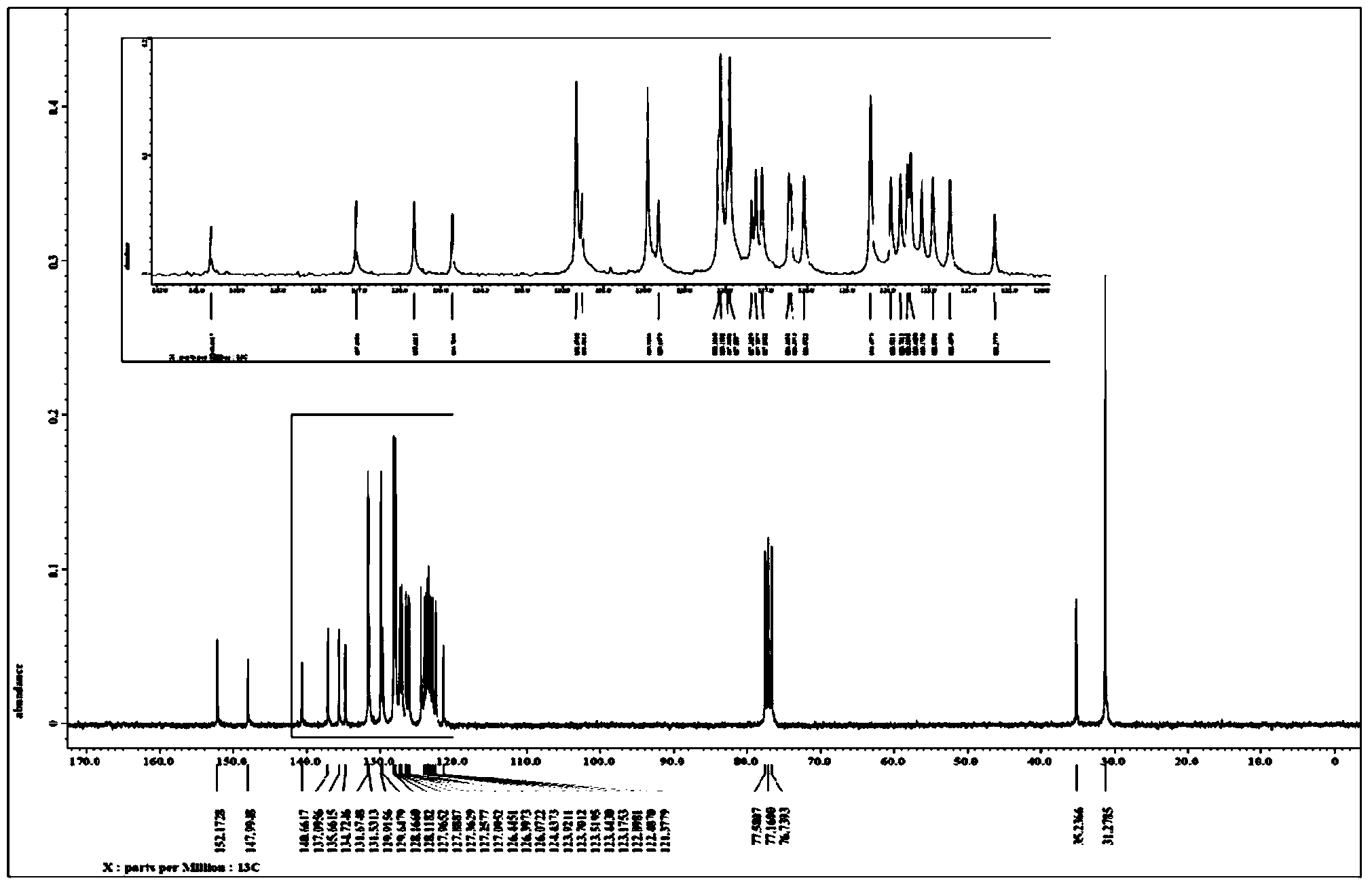
The invention relates to a synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds, wherein the synthesis method comprises the steps: in an organic solvent system, synthesizing by taking a 1-aryl methylene indole compound as a basic raw material in the presence of a palladium catalyst, an oxidizing agent and an auxiliary additive, and post-treating the reaction liquid to obtain the 6-hydroisoindolo[2,1-alpha]indole compounds. According to the invention, starting from a 1-aryl methylene indole compound, in the presence of the palladium catalyst and the oxidizing agent, an intramolecular double-carbon hydrogen bond activation reaction is realized, and various 6-hydroisoindolo[2,1-alpha]indole compounds are synthesized at high yield. Indole raw materials which are simple and easy to synthesize and a catalytic amount of palladium are used in the reaction, the universality of a substrate is good, and the reaction operation is simple and convenient and high in efficiency. The obtained product can be used as a medical intermediate or a fluorescent luminescent material.
Owner:YANGZHOU UNIV
Synthesis method of nuciferine or derivative thereof, nuciferine derivative and application of nuciferine derivative
PendingCN114751860ARapid expansionEasy to getOrganic chemistryMetabolism disorderPharmacologic therapyHyperlipidemia
The invention relates to the technical field of medicine synthesis, and provides a synthetic method of nuciferine or a derivative thereof, the nuciferine derivative and application of the nuciferine derivative. The preparation method comprises the following steps: carrying out Schotan-Baumann reaction on a compound with a structure as shown in a formula IV and R-X, and then activating through a carbon-hydrogen bond to obtain nuciferine or a derivative thereof. Furthermore, the compound with the structure shown in the formula IV is obtained by taking 2-bromophenylacetic acid as an initiator and carrying out acylating chlorination reaction, nucleophilic substitution reaction, Bischler-Napieralski reaction and reduction reaction. The synthesis method provided by the invention has the advantages of simple steps and easily available raw materials, and the carbon-hydrogen bond activation method is efficient, direct and flexible, and is very suitable for synthesis of nuciferine molecules. The nuciferine can be synthesized, and the nuciferine can be flexibly subjected to structural modification, so that a series of nuciferine derivatives are obtained, and a solid foundation is provided for developing nuciferine drugs for treating obesity and hyperlipidemia.
Owner:HUAQIAO UNIVERSITY +1
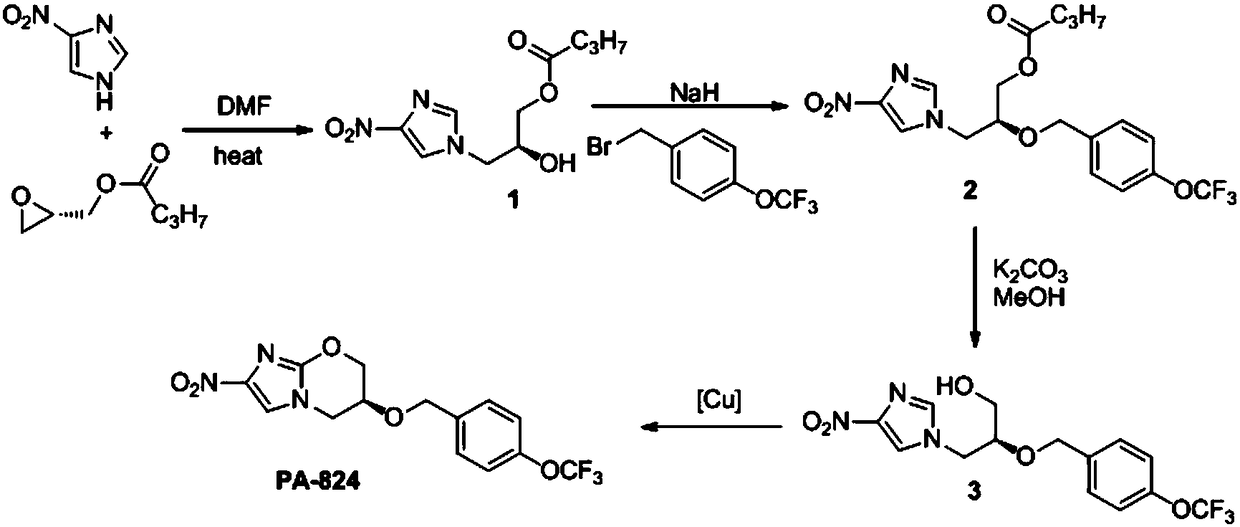
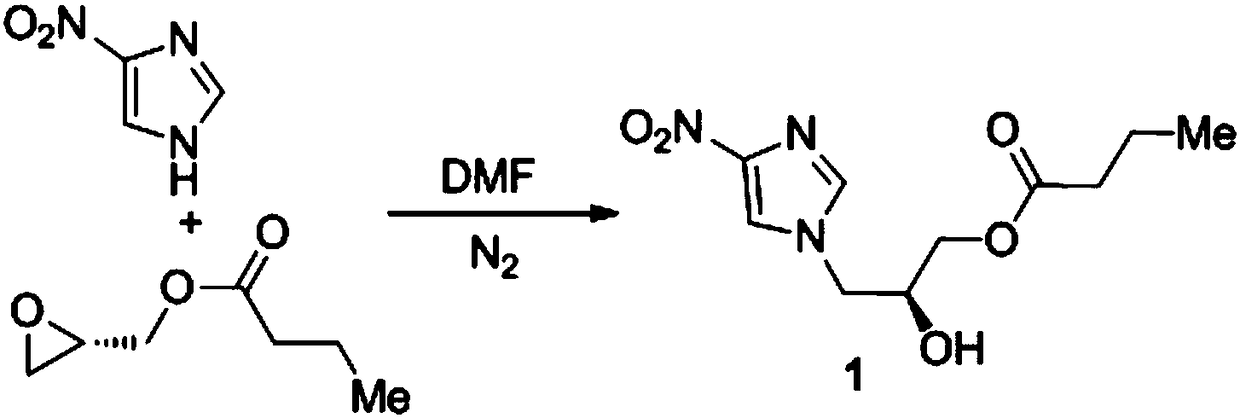
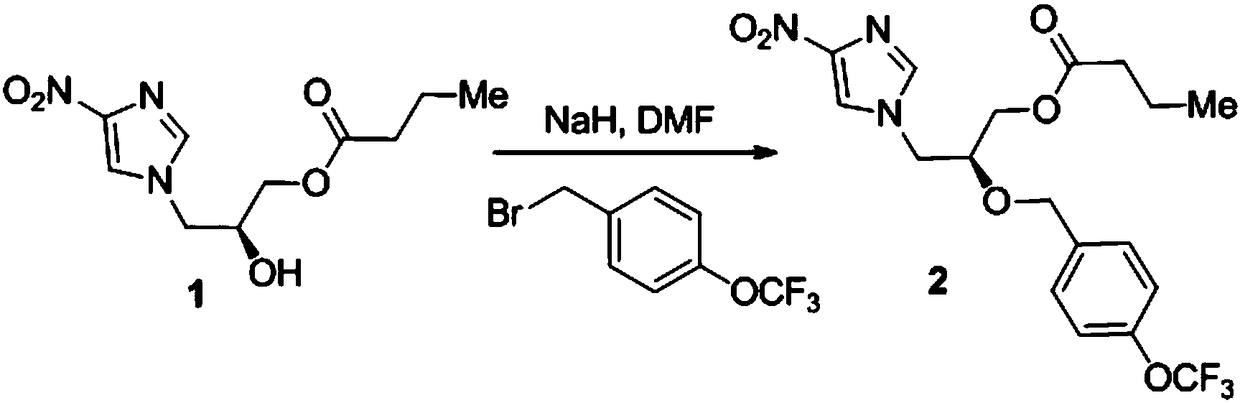
Preparation method of anti-tuberculosis candidate drug pa-824
ActiveCN106632393BReduce usageEasy to operateAntibacterial agentsOrganic chemistry methodsNitroimidazoleAlkyl transfer
The invention relates to a synthetic method for an antituberculous candidate drug namely PA-824. The method realizes synthesis of the PA-824 through four steps of reactions which comprise a nucleophilic ring-opening reaction, an o-alkylation reaction, an ester deprotection reaction and a transition metal catalyzed carbon-hydrogen bond activated alkoxylation reaction. The preparation method provided by the invention has the advantages of cheap and easily available raw materials needed in the whole preparation process, avoids using an explosive raw material namely 2,4-dinitroimidazole, has simple and easily controllable operation, is convenient to purify, and facilitates to large-scale production.
Owner:JINGCHU UNIV OF TECH +1
Preparation method of 3-nitroso substituted indole derivative
The invention belongs to the technical field of organic synthesis and particularly relates to a preparation method of a 3-nitroso substituted indole derivative. The preparation method comprises the following steps: mixing N-nitrosoaniline as shown in a formula II, acyl sulfur ylide as shown in a formula III, a catalyst, silver salt and a solvent, performing carbon-hydrogen bond activation reaction, adding protonic acid and performing cyclizing and cascaded nitroso migration reaction, wherein the catalyst is a compound of transition metal and the transition metal is at least one of rhodium, ruthenium, cobalt, iridium and palladium. The structural formula of the 3-nitroso substituted indole derivative prepared by the preparation method is as shown in a formula I. By the preparation method provided by the invention, synthesis of the indole derivative can be realized by mixing the raw materials through a one-pot method, so the preparation process is simple and easy to control.
Owner:ZHENGZHOU UNIV
Deuterated synthesis method of indole compound
ActiveCN111533676AMild reaction conditionsEasy to getIsotope introduction to heterocyclic compoundsPtru catalystReaction temperature
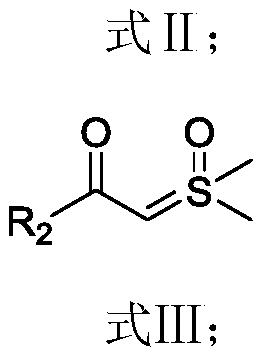
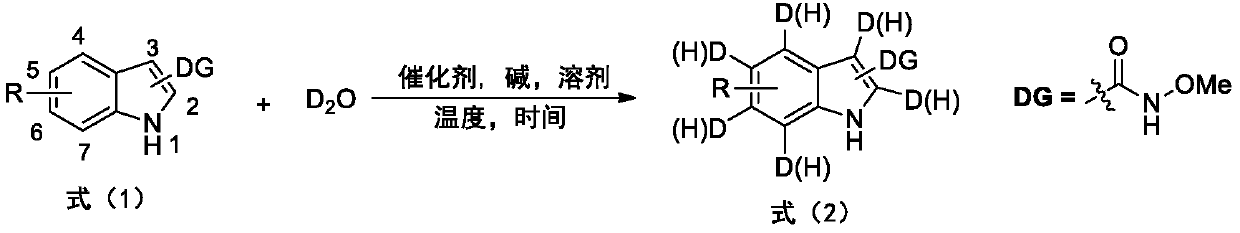
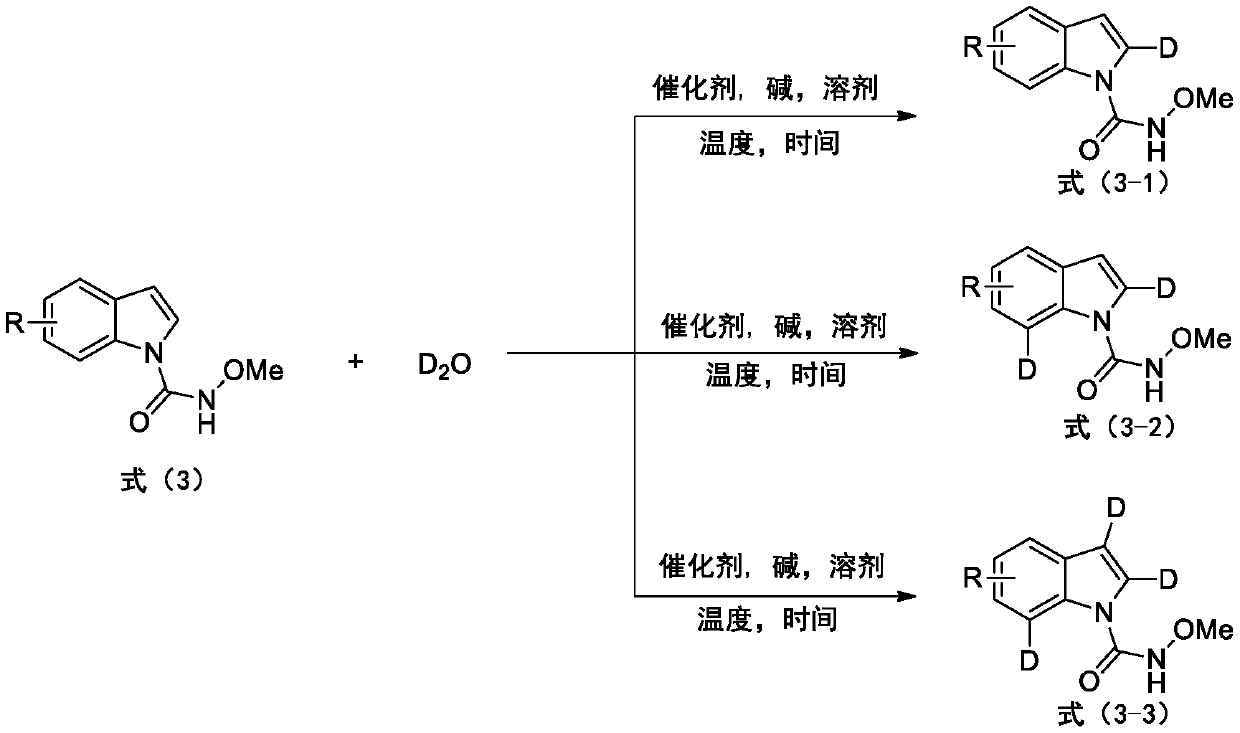
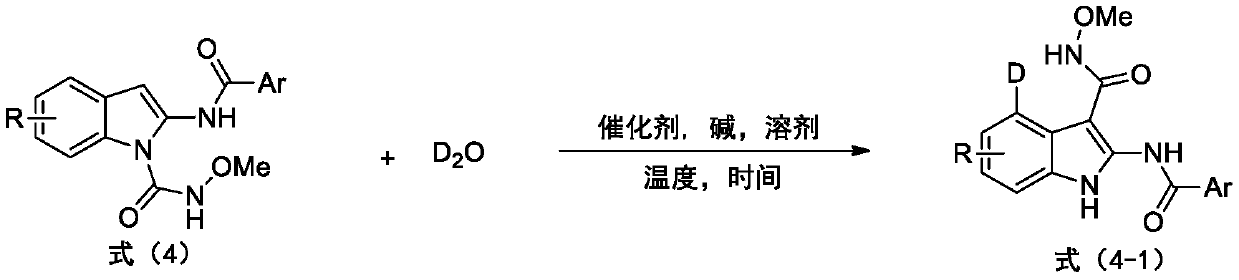
The invention provides a deuterated synthesis method of an indole compound, which comprises the following steps: carrying out hydrogen-deuterium exchange on the indole compound through a carbon-hydrogen bond activation reaction guided by a guide group DG at a proper reaction temperature for proper time under the mixed reaction of heavy water, a catalyst, alkali and a solvent to generate a deuterated indole product. The method provided by the invention is mild in reaction condition, simple in process, low in cost, good in deuteration position selectivity and high in yield and deuteration rate,moreover, relatively cheap heavy water is used as a deuterium source, so that the use of an expensive deuterium source in the preparation process of the deuterated compound is effectively avoided, andthe preparation cost of the deuterated compound can be further reduced.
Owner:ZHEJIANG UNIV
Method for synthesizing o-nitroacetophenone compound
InactiveCN101985424BGood substrate adaptabilityOrtho nitrificationOrganic chemistryOrganic compound preparationOrtho positionNitration
The invention provides a method for synthesizing o-nitroacetophenone compound, relating to a method for synthesizing organic compound. The invention takes acetophenone (can contain various substituent groups) as raw material directly, carbonyl group is converted into hydroxyimino, then palladium is taken as catalyst, carbon-hydrogen bond activation single nitration on the adjacent position of hydroxyimino is realized in the presence of oxidant and nitration agent, and finally hydroxyimino is hydrolyzed into corresponding ketone again under the action of acid, thus realizing nitration of adjacent position of carbonyl group of acetophenone and obtaining a series of o-nitroacetophenone compounds. The invention is safe and environmentally friendly, no waste gas or waste water is produced; adaptability of substrate is good, adjacent position nitration on various substituent groups can be realized; regioselectivity of nitration reaction is good; various nitroacetophenones are directly takenas raw materials, and reaction process is simple, thus being a new approach for synthesizing o-nitroacetophenone compound containing substituent groups.
Owner:ZHEJIANG UNIV OF TECH
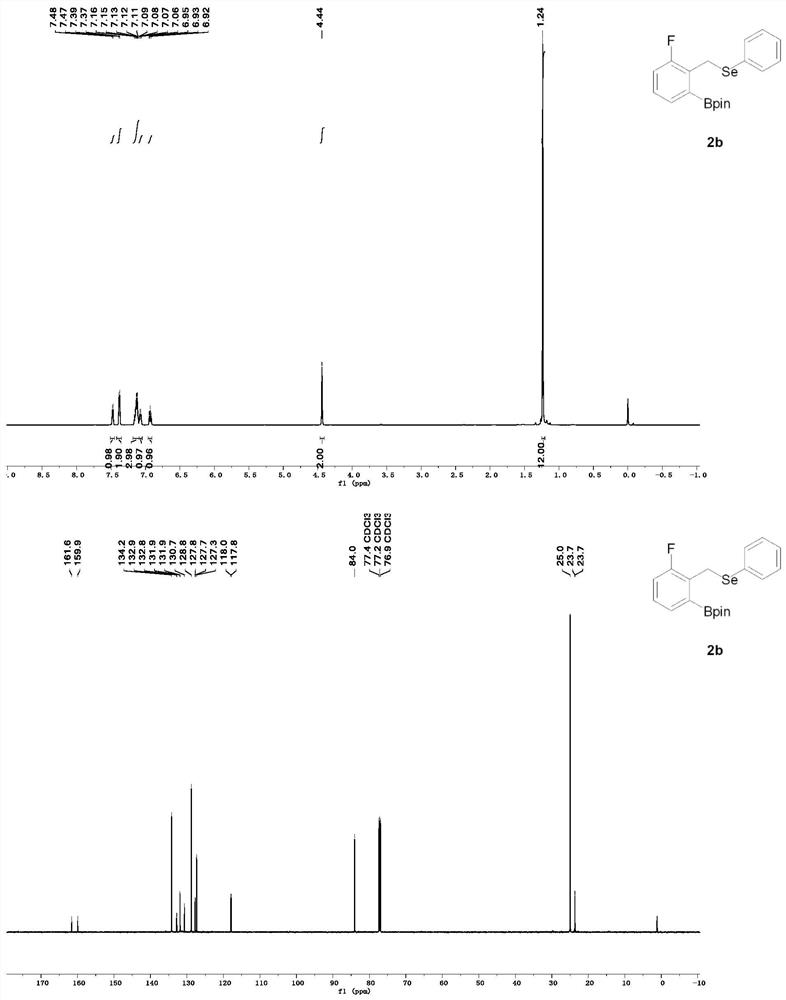
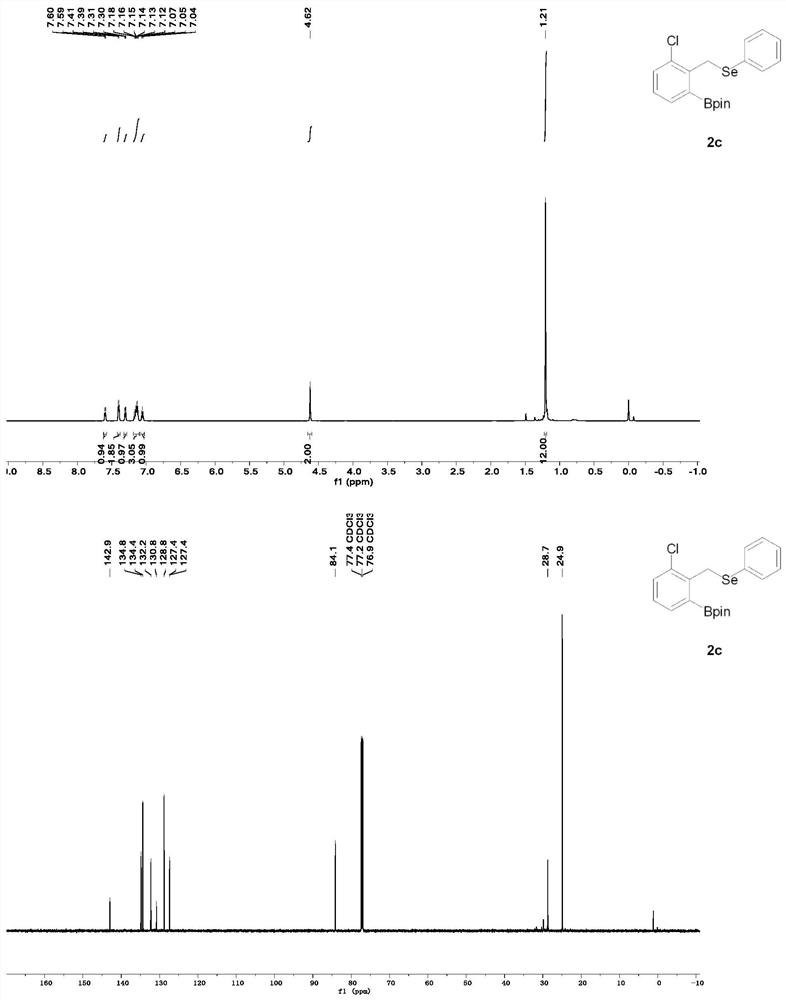
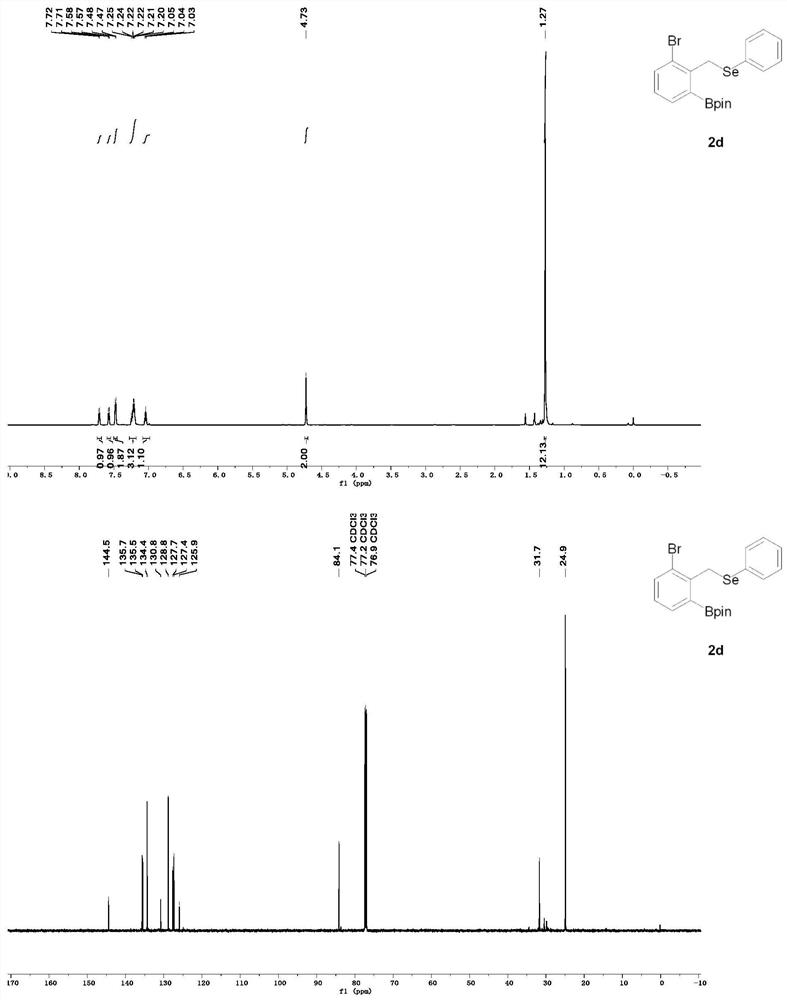
A method for rapid preparation of benzylselenide compounds based on selenium-directed carbon-hydrogen bond boronation
The invention provides a method for rapidly preparing benzyl selenium compounds based on selenium-directed carbon-hydrogen bond boronation, belonging to the field of organic synthesis. Under relatively mild conditions, the present invention uses selenium-containing organic compounds (benzyl phenyl selenium derivatives) as raw materials, cheap pinacol borane as the boron source, and double boron pinacol ester as the additive. The transition metal iridium is used as a catalyst to catalyze the reaction of carbon-hydrogen bond activation at the ortho position, thereby preparing a series of boronated products of organoselenium compounds. The advantages of the present invention are: the yield of the product is high, the reaction conditions are warm, and the organic selenium boride can be conveniently and quickly converted into a new organic selenium compound, and the spatial configuration is kept unchanged, and the good activity is maintained for the synthesis of organic selenium. new method. Therefore, the present invention provides an effective solution for the industrial production of other high-value compounds containing this structure in the future.
Owner:SOUTHWEST PETROLEUM UNIV
A kind of bipyrene and its preparation method and use
ActiveCN105037106BRaw materials are cheap and easy to getEasy to prepareOrganic chemistryOrganic compound preparation1-bromopyreneCoupling
The invention relates to dipyrenol, a preparation method and an application thereof. The preparation method includes the steps of performing a coupling reaction with 1-bromopyrene and 1-hydroxypyrene as raw materials under catalysis of palladium, and treating the product after the reaction is finished to obtain the dipyrenol. The dipyrenol is a new compound which is not reported before and has excellent application value in the field of photoresists. Compared with conventional methods for preparing the compound, the preparation method relates to carbon-hydrogen bond activation and coupling of an aromatic compound. A palladium catalyst is easy to obtain. The reaction method is simple and the reaction conditions are easy to reach. The preparation method is high in application value and commercial value.
Owner:VALIANT CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
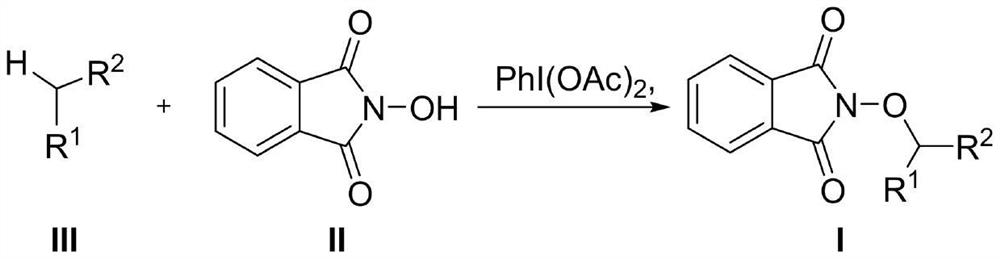
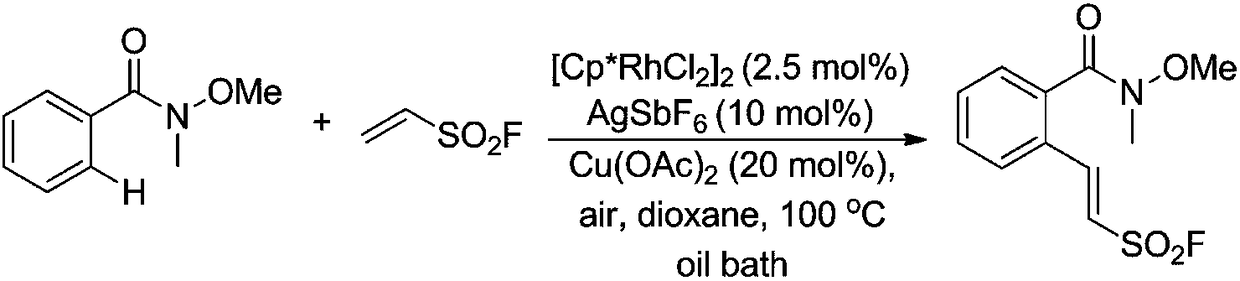
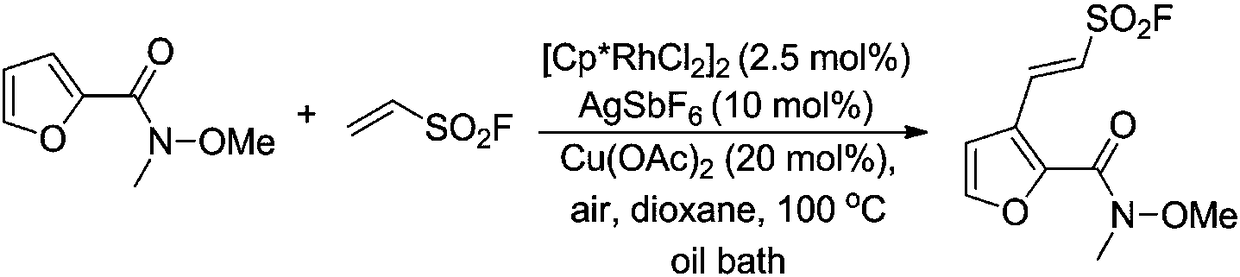
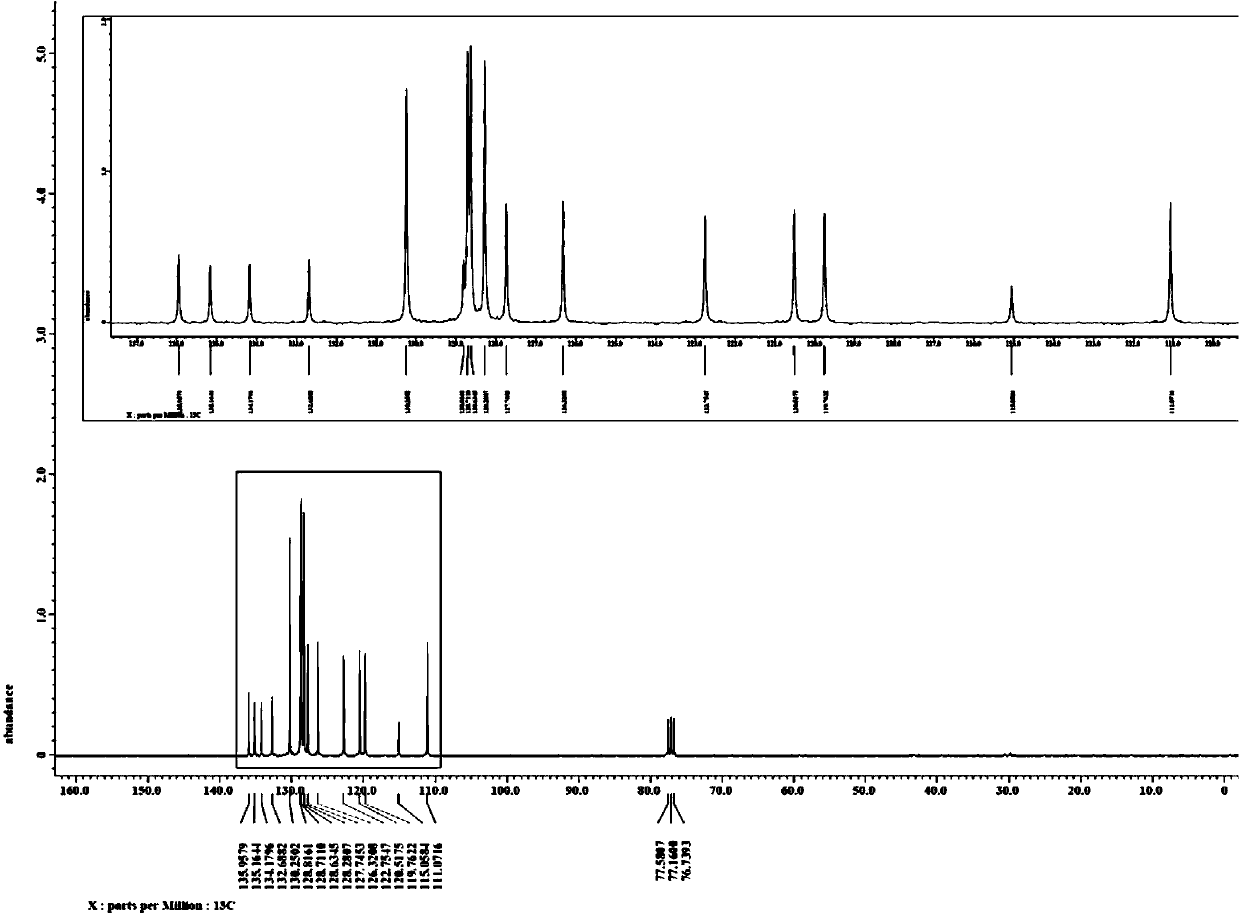
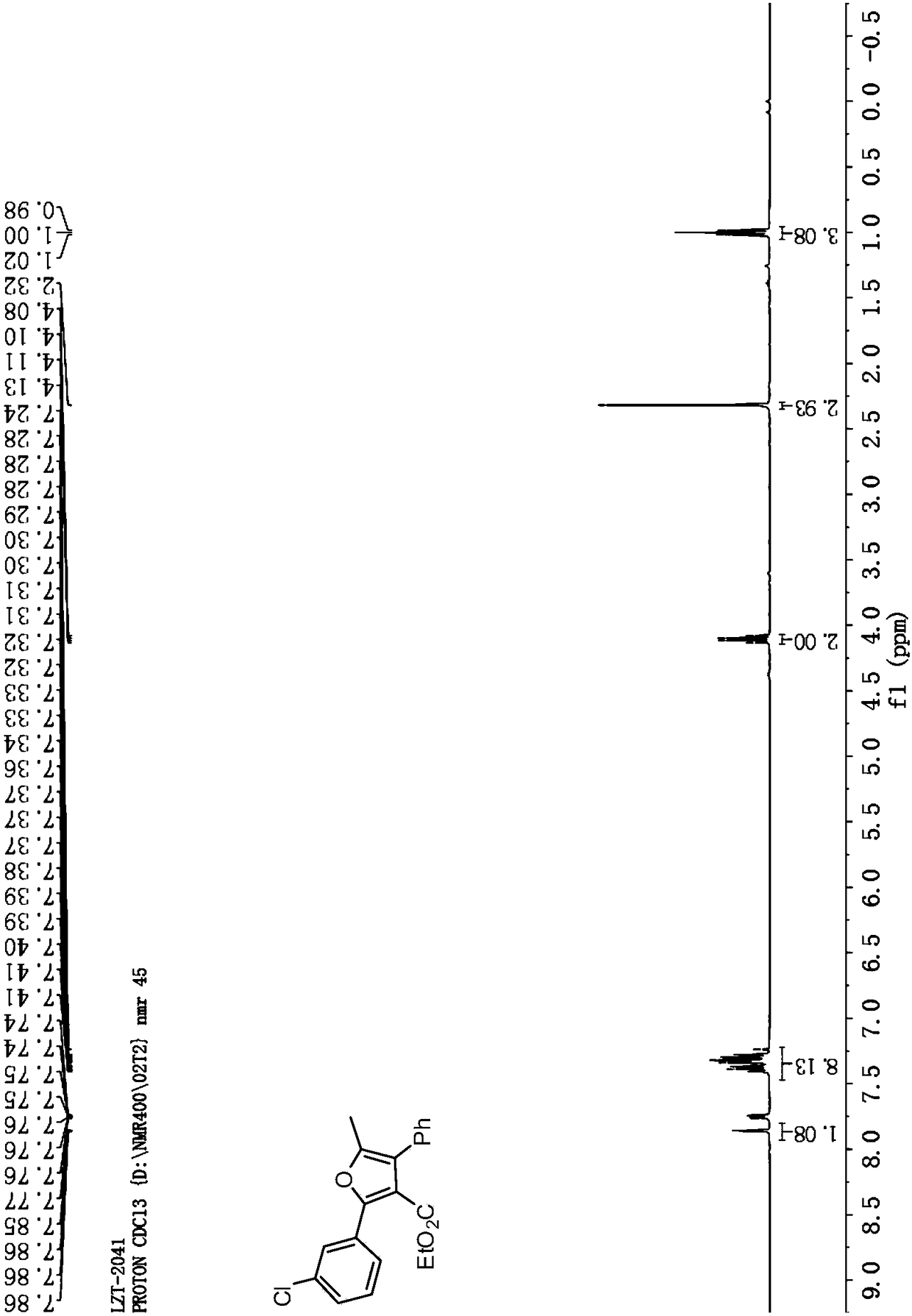

![Synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds Synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds](https://images-eureka.patsnap.com/patent_img/db8fc065-2f3d-4e3d-9e65-01f0032342d3/HDA0003041030830000011.png)
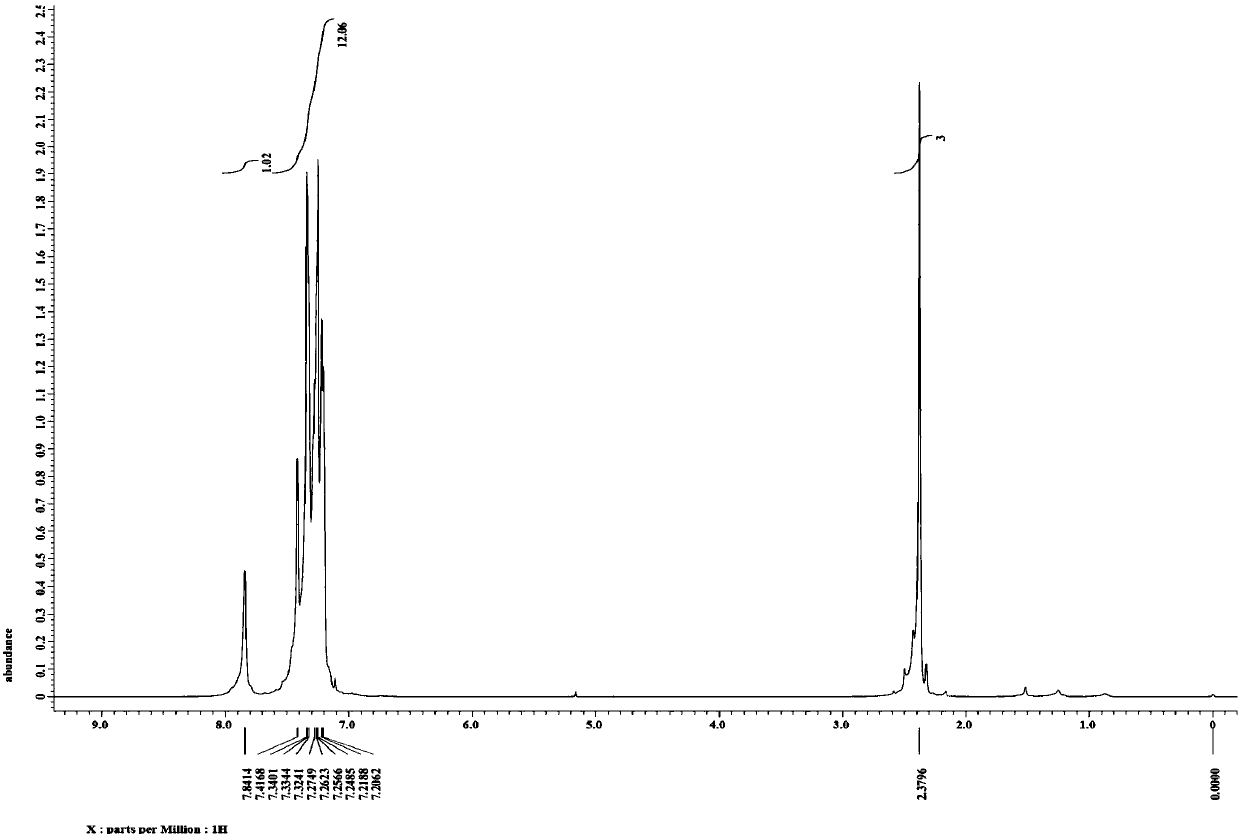
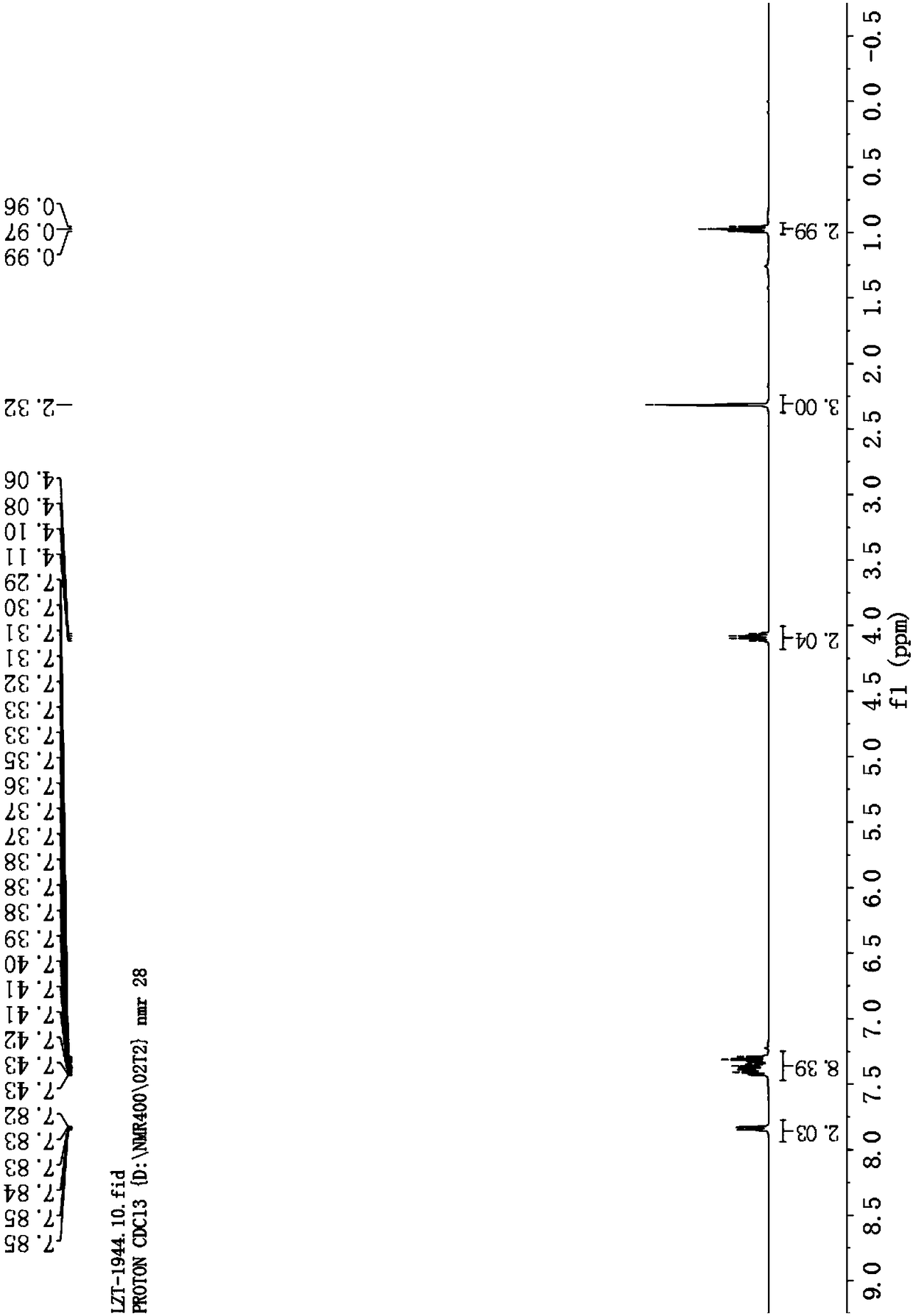
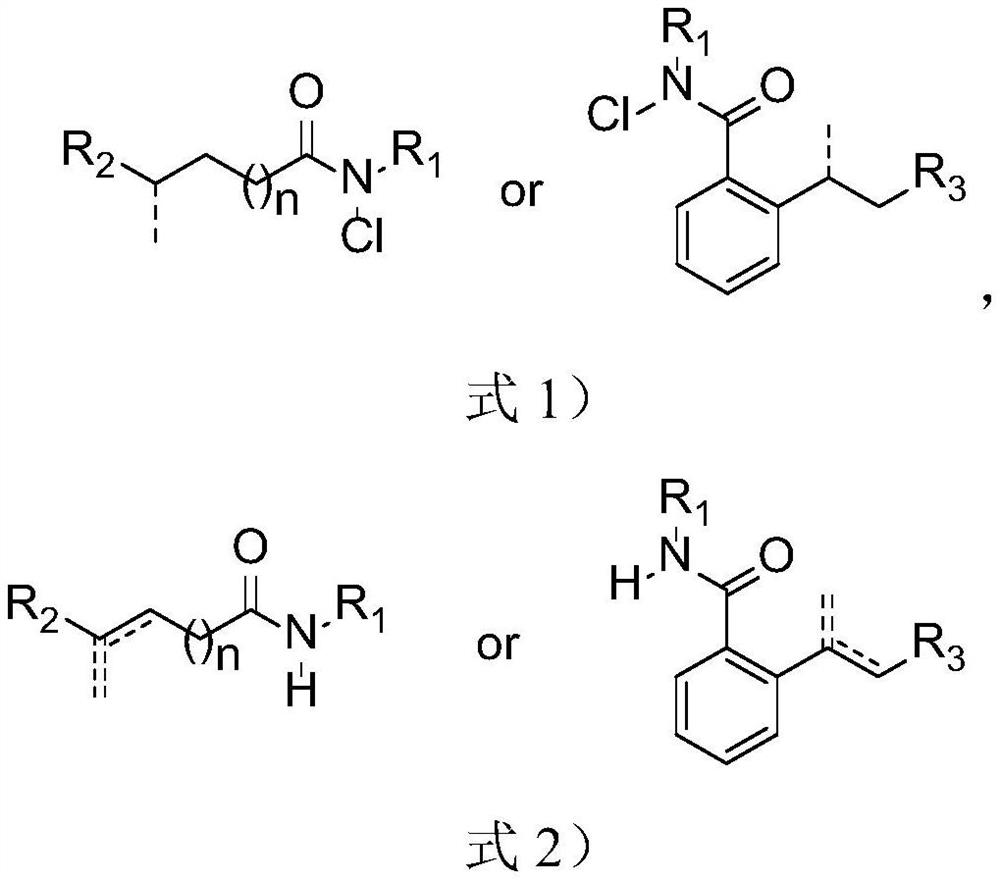
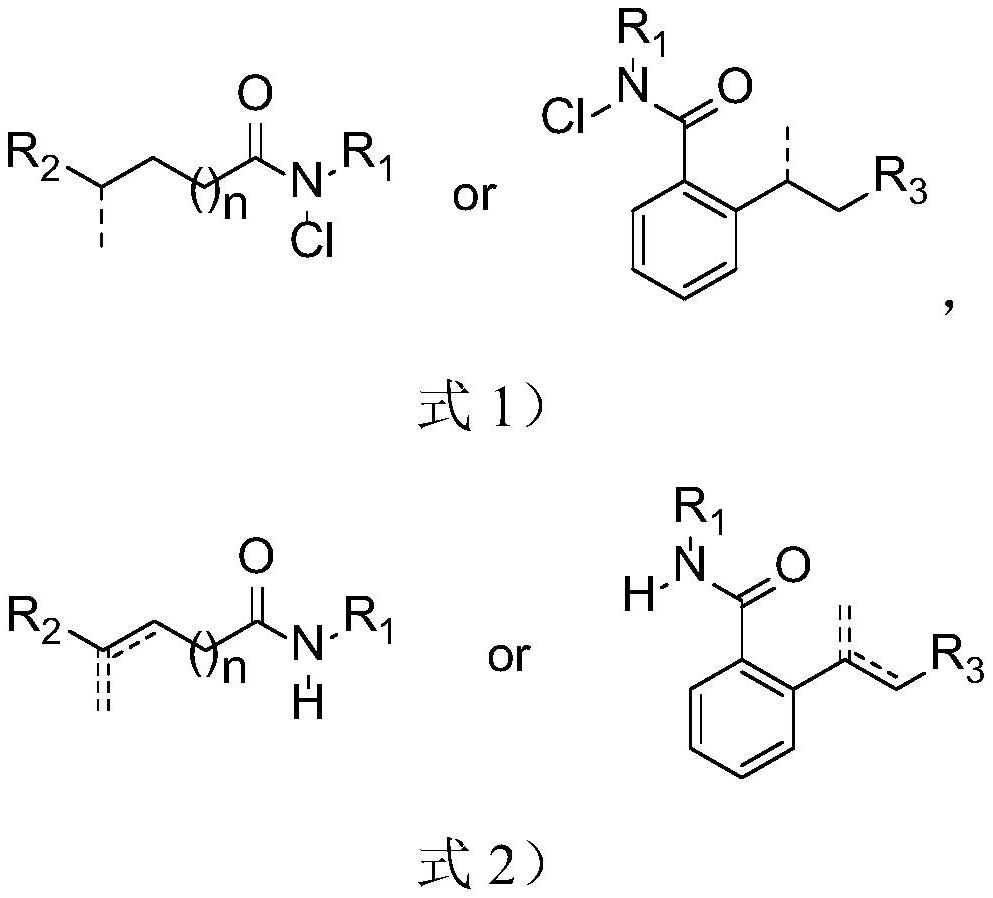
![Synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds Synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds](https://images-eureka.patsnap.com/patent_img/db8fc065-2f3d-4e3d-9e65-01f0032342d3/HDA0003041030830000012.png)
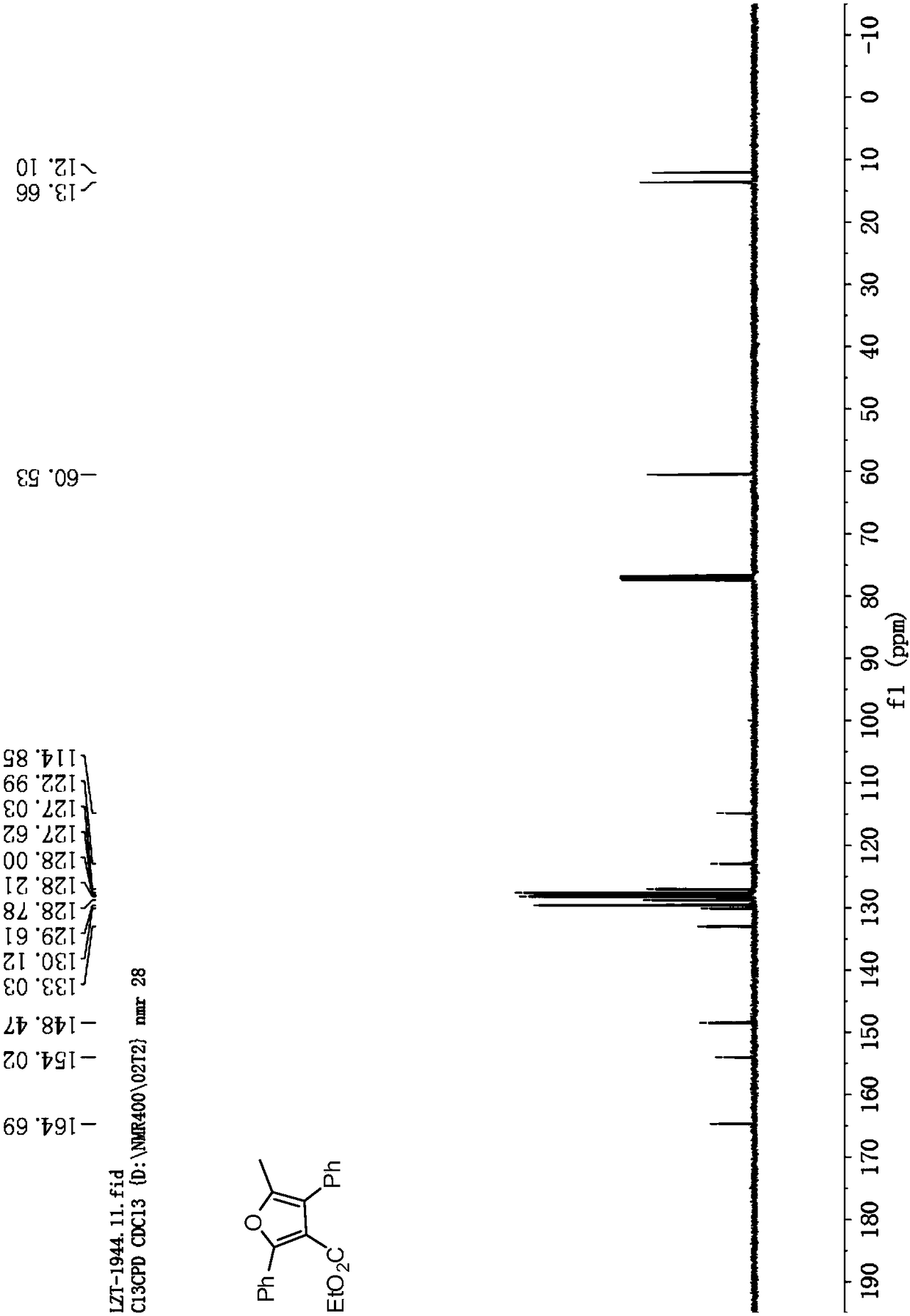
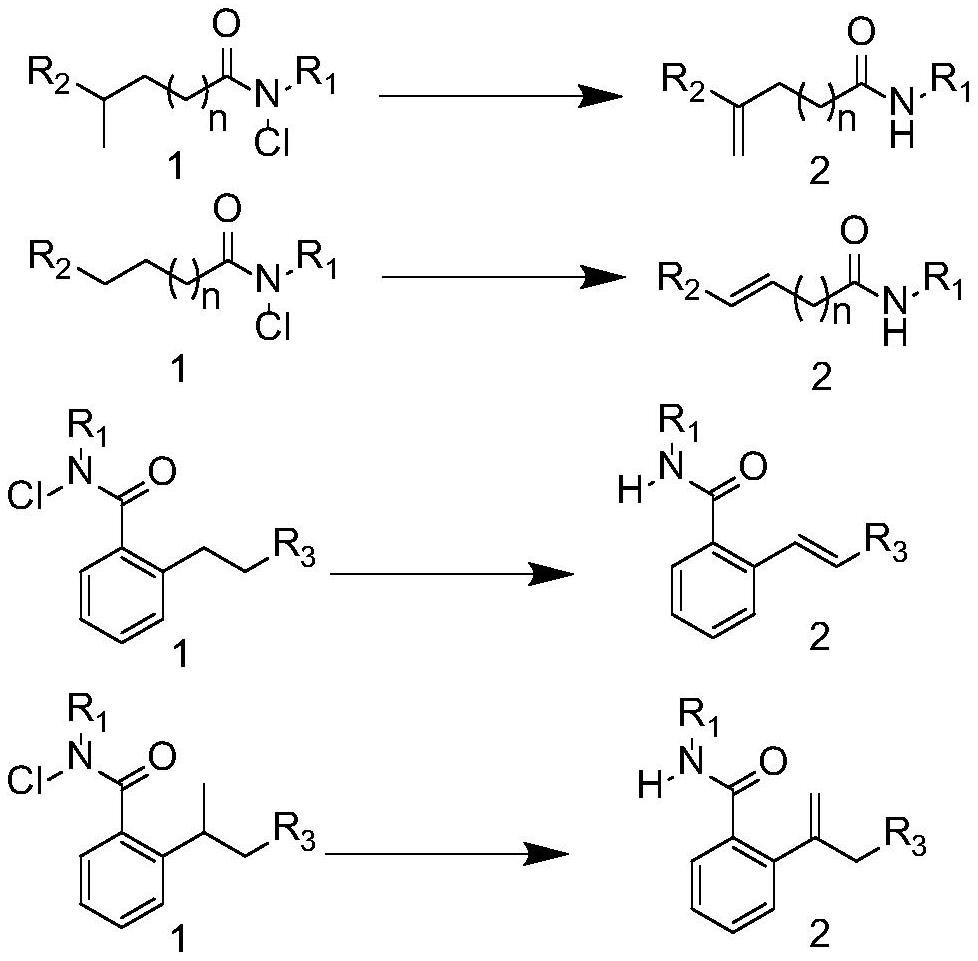
![Synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds Synthesis method of 6-hydroisoindolo[2,1-alpha]indole compounds](https://images-eureka.patsnap.com/patent_img/db8fc065-2f3d-4e3d-9e65-01f0032342d3/FDA0003041030810000011.png)