Synthesis method of nuciferine or derivative thereof, nuciferine derivative and application of nuciferine derivative
A technology of a lotus alkaloid derivative and a synthesis method, which is applied in the directions of drug combination, organic chemistry, metabolic diseases, etc., can solve the problems such as no effective chemical synthesis method of lotus alkaloid or its derivatives, and achieves simple, efficient and easy synthesis steps. Obtain, respond efficiently
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The invention provides a kind of synthetic method of lotus leaf base or its derivative, comprising the following steps:
[0062] (1) the compound with the structure shown in formula IV, R-X and the basic catalyst are mixed to carry out the Schotten-Baumann reaction to obtain the compound with the structure shown in formula V; in the R-X, R is an aliphatic hydrocarbon group, an aromatic hydrocarbon group or a Acyl, X is halogen;
[0063]
[0064] (2) mixing the compound with the structure shown in formula V, the phosphine ligand compound, the basic reagent and the catalyst to carry out a carbon-hydrogen bond activation reaction to obtain lotus leaf base or its derivative;
[0065] Said lotus leaf base or its derivative has the structure shown in formula VI:
[0066]
[0067] When R is a methyl group, the compound of the structure shown in formula VI is salicylic acid, and when R is another group, the compound of the structure shown in formula VI is a salicylic aci...
Embodiment 1
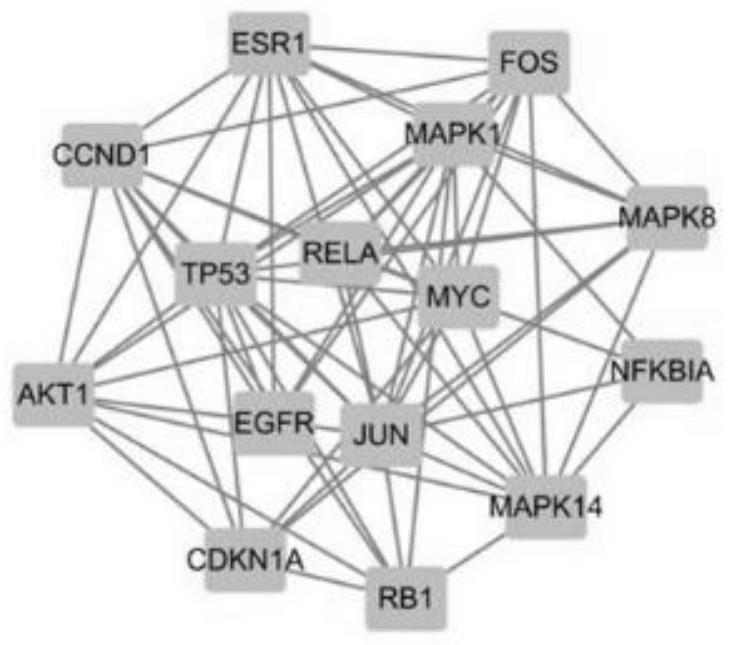
[0122] Network Pharmacology and Molecular Docking Technology
[0123] 1. Materials and methods
[0124] Screening of drug components and targets: TCMSP (Traditional Chinese Medicine System Pharmacology Database and Analysis Platform) software systematically analyzes pharmacokinetics, pharmacodynamics, omics, etc. Provides a platform for pharmacology and validation studies. At the same time, it also provides new research methods for target discovery and new drug development. The invention retrieves the chemical constituents of lotus leaves according to the inclusion of the TCMSP database, a unique system pharmacology platform of traditional Chinese medicine. Taking the two conditions of oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 as the thresholds, the corresponding compounds and corresponding targets were screened out. The main steps are as follows:
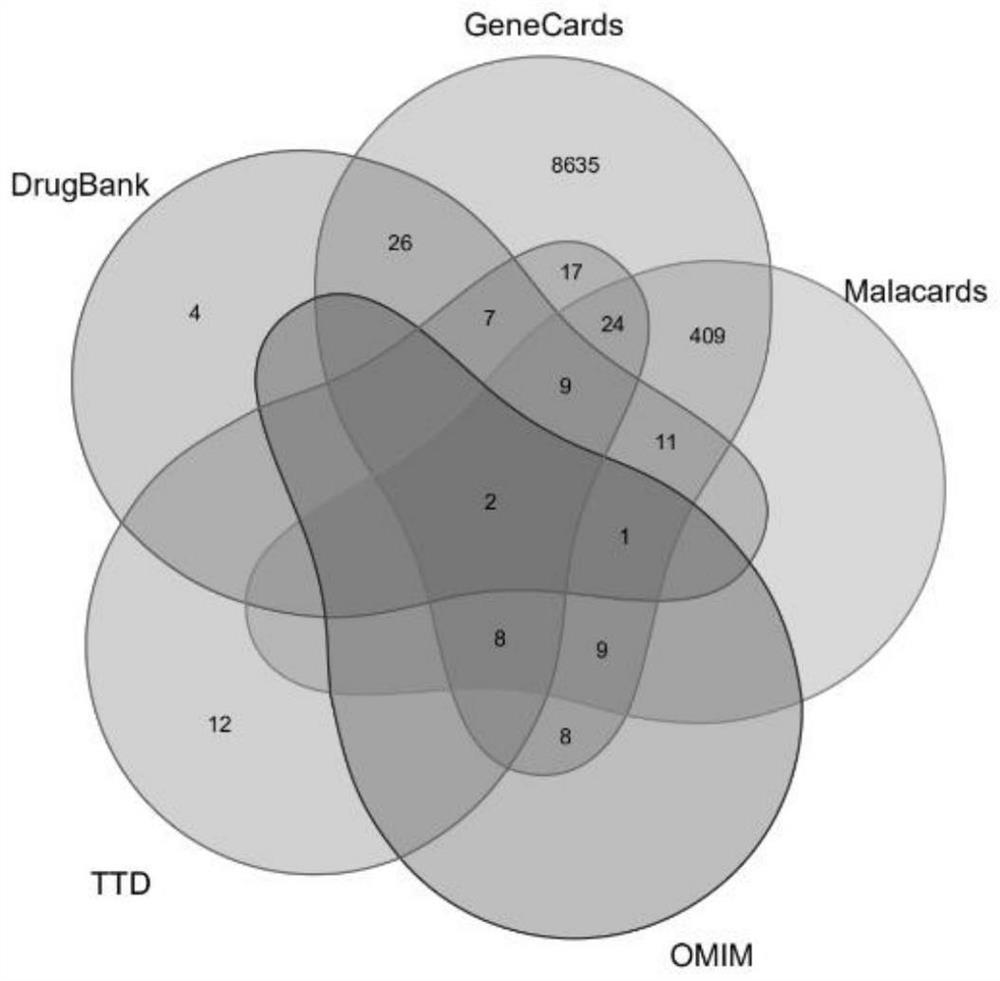
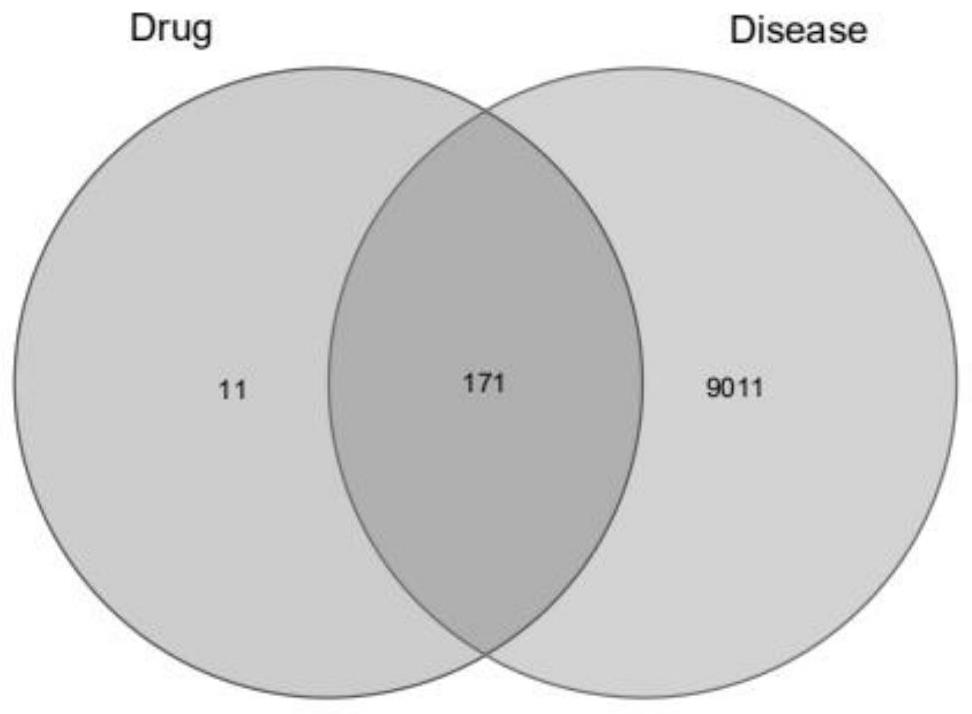
[0125] Target acquisition of disease-related effects: The following databases were searched and screened ...
Embodiment 2
[0152] Preparation of benzoyl salicylate (referred to as compound 6a)
[0153] The synthetic route of described benzoyl lotus leaf base is as shown in formula 1:
[0154]
[0155] At 0 °C, oxalyl chloride (30 mmol, 2.54 mL) was added to 2-bromophenylacetic acid (30 mmol, 6.45 g) dissolved in DCM (30 mL), then 0.6 g of DMF was added, and the reaction was performed at 30 °C for 2 h. After completion, rotary evaporation was carried out at 35°C to obtain compound 1 (10.3 g, 92%).
[0156] 3,4-Dimethoxyphenethylamine (30 mmol, 5.06 mL) and triethylamine (33 mmol, 4.58 mL) were dissolved in DCM (40 mL), then compound 1 (20 mmol, 10 g) was added at 0°C, The reaction was carried out at 35°C for 12h. After the reaction was completed, the reaction was quenched with 40 mL of brine, and then extracted with 100 mL of DCM. After drying with 10 g of anhydrous sodium sulfate, the anhydrous sodium sulfate was removed by filtration, and then rotary-evaporated at 35 °C to obtain compound 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com