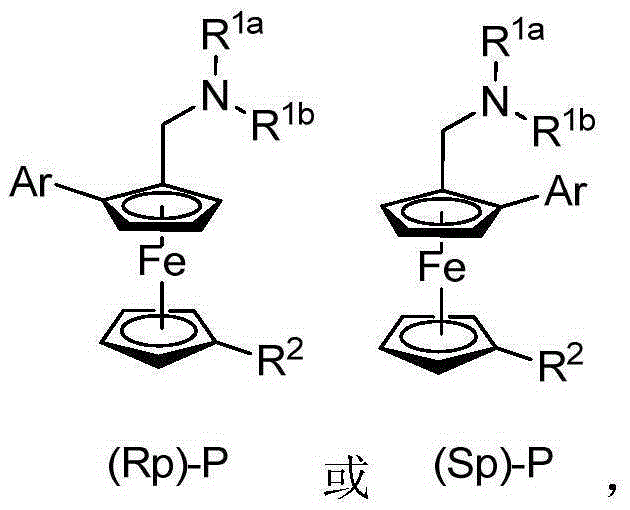
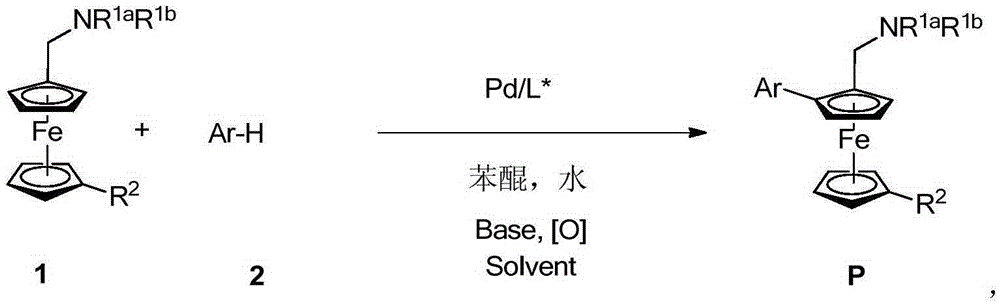
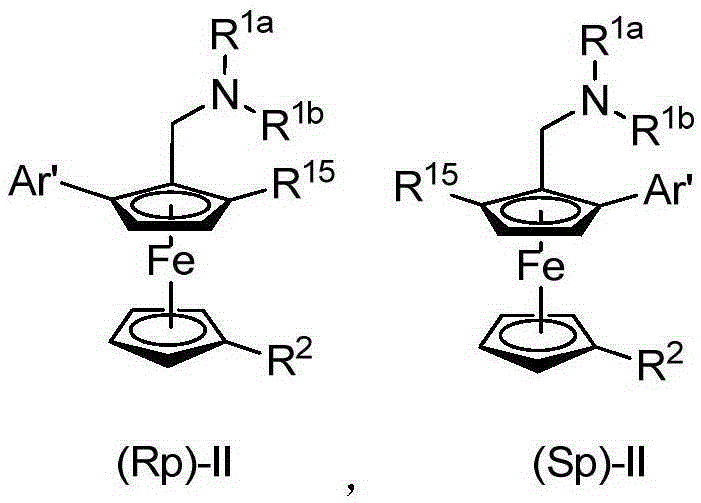
Planar chiral ferrocene compound, synthetic method and application
A technology of chiral ferrocene and synthesis method, which is applied in the field of planar chiral ferrocene compounds, can solve the problems of lack of efficient synthesis methods, achieve excellent yield and enantioselectivity, mild reaction conditions, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Partial amino acid screening results
[0039]
[0040] In the reaction formula, Pd(OAc) 2 Represents palladium acetate, ligand represents the chiral amino acid ligand, mol describes the mole, K 2 CO 3 Represents potassium carbonate, equiv. represents equivalent, DMA represents N,N-dimethylacetamide, air represents air, and T represents temperature.
[0041] Add the corresponding benzofuran 2a and DMA (1.5mL) to the dry Schlenk reaction flask, followed by amino acid (0.06mmol), palladium acetate (0.03mmol), potassium carbonate (0.45mmol), benzoquinone (0.03mmol) , water (1.2 mmol) and ferrocene 1a (0.3 mmol). The reaction was heated under air atmosphere. After the reaction was completed, the reaction was quenched with saturated sodium bicarbonate and extracted with ethyl acetate. The organic phases were combined, washed successively with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent residue was removed under ...
Embodiment 2
[0045] Embodiment 2: the asymmetric synthesis of planar chiral ferrocene compound (Rp)-P or (Sp)-P
[0046] Reaction 1
[0047]
[0048] Reaction 2
[0049]
[0050] In reaction formula 1, Pd(OAc) 2 Represents palladium acetate, Boc-L-Ile-OH represents (S)-N-tert-butoxycarbonyl isoleucine, mol describes mole, equiv. describes equivalent, K 2 CO 3 stands for potassium carbonate, BQ stands for p-quinone, H 2 O represents water, DMA represents N,N-dimethylacetamide, and air represents air; in Reaction Formula 2, Boc-D-Ile-OH represents (R)-N-tert-butoxycarbonylisoleucine.
[0051] Add palladium acetate (6.7mg, 0.03mmol), Boc-L-Ile-OH (13.9mg, 0.06mmol) or Boc-D-Ile-OH (13.9mg, 0.06mmol), potassium carbonate to a dry Schlenk reaction flask (62.2mg, 0.45mmol), BQ (3.2mg, 0.03mmol), water (21.6mg, 1.2mmol) and DMA (1.5mL). The corresponding ferrocene substrate 1 (0.3 mmol) and heteroarene 2 (0.6 mmol) were added. Heated to 80°C under air atmosphere to react. After the ...
Embodiment 3
[0132] Embodiment 3: the synthesis of (Rp)-II or (Sp)-II
[0133] Reaction 1
[0134]
[0135] Reaction 2
[0136]
[0137] t-BuLi means tert-butyl lithium, EX means benzophenone or diphenylphosphine chloride or trimethylchlorosilane or diphenyl disulfide, Et 2 O represents ether.
[0138] Under argon protection, add (R p )-S-1 or (S p )-S-1(1equiv.) and dry diethyl ether (0.06mol / L), cooled to -78°C, added t BuLi (1.5 equiv.). The reaction was stirred at -78°C for 5 hours. Then, a diethyl ether solution (2.0 equiv. 0.2 mol / L) of the electrophile was added dropwise to the reaction system. The reaction was returned to room temperature, stirred overnight, and reacted for 2-20 hours. The reaction mixture was quenched by adding ice water and extracted with ethyl acetate. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and filtered. The solvent was removed under reduced pressure and purified by column chromatography (ethylaceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com