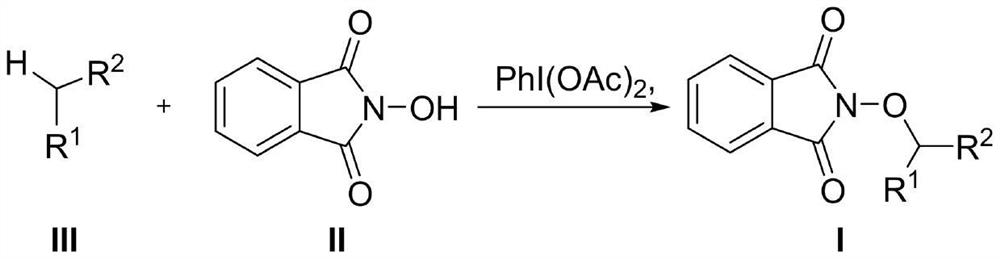Carbon-hydrogen bond activation method for non-metal participated inert alkane
An activation method, a non-metallic catalyzed technology, applied in the field of non-metallic carbon-hydrogen bond activation of inert alkanes, can solve the problems of no activation group and high carbon-bond bond energy, and achieve simple post-treatment, mild reaction conditions, The method is simple and efficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The reaction formula of Example 1, the specific structures of compounds III-1, II-1 and product I-1 used are as follows
[0027]
[0028] The specific steps are: iodobenzene acetate (CAS: 3240-34-4, 2mmol) and N-hydroxyphthalimide (CAS: 524-38-9, 1mmol) and the corresponding substrate (2mmol) are added to 2 mL of dichloromethane, stirred at room temperature for 2 hours. Thin-layer chromatography (TLC) monitors that the reaction raw materials are consumed, the reaction system is dried by a rotary evaporator, and the product is obtained by silica gel column chromatography (eluent PE:EA, 50:1 to 20:1). The compound shown in I-1 was obtained, and its product was identified by NMR (H spectrum, C spectrum) and high resolution mass spectrum.
[0029]
[0030] Product I-1 was a yellow solid. (Yield=45%).m.p.98–100℃. 1 H NMR (400MHz, CDCl 3 )δ7.82(m,2H),7.76–7.71(m,2H),4.09(q,J=6.4Hz,1H),1.23(d,J=6.4Hz,3H),1.09(s,9H). 13 CNMR (101MHz, CDCl 3 )δ163.4, 133.3, 128.0, 12...
Embodiment 2
[0033] The method used in the embodiment of preparing other compounds of the present invention (compound I-2 to compound I-9) is identical with embodiment 1, and reaction condition is as follows: compound III (2mmol, 2 equivalents), compound II (1mmol, 1 equivalent) , Iodobenzene acetate (2mmol, 2 equivalents) was dissolved in 2mL of dichloroethane, stirred at room temperature for 2 hours.
[0034] The structures and data of each product obtained are characterized as follows:
[0035]
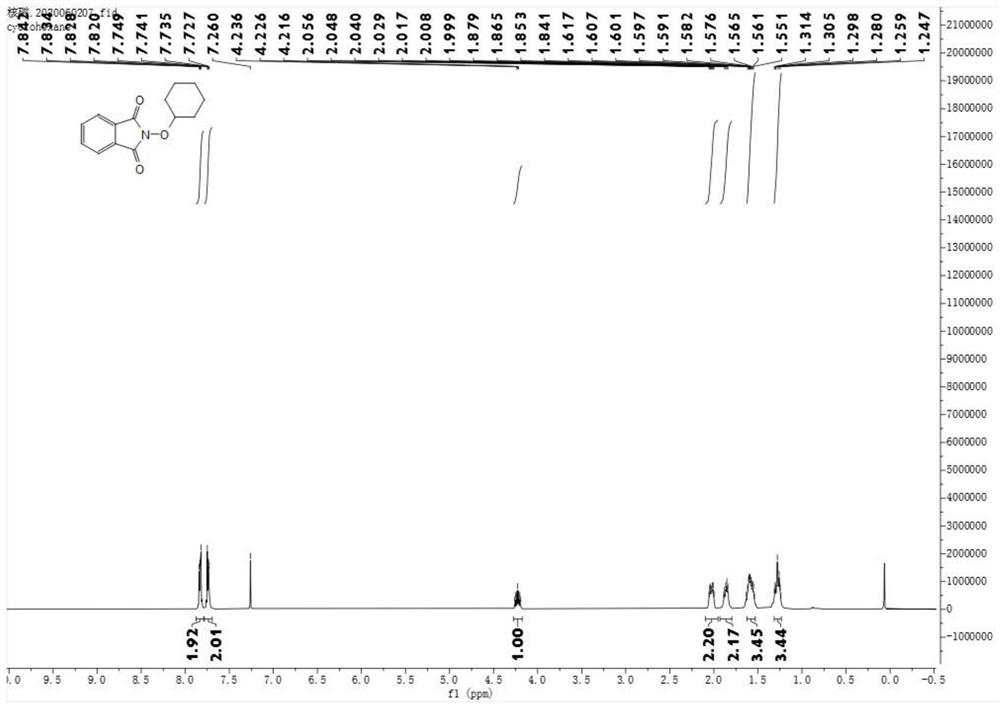
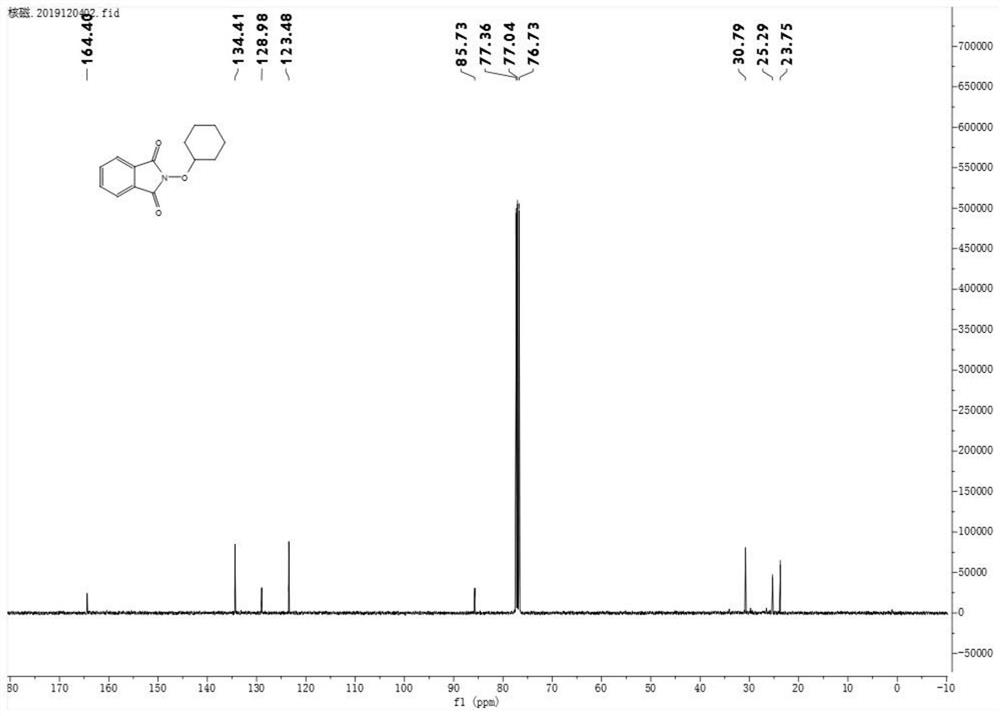
[0036] Product Ⅰ-2 is a yellow solid (yield 61%)
[0037] m.p.116–118°C. 1 H NMR (400MHz, CDCl 3 )δ7.85–7.82(m,2H),7.75–7.73(m,2H),4.26–4.19(m,1H),2.06–2.00(m,2H),1.90–1.83(m,2H),1.63– 1.54(m,3H),1.25–1.28(m,3H). 13 C NMR (101MHz, CDCl 3 )δ164.4, 134.4, 129.0, 123.5, 85.7, 30.8, 25.3, 23.8.
[0038] Product Ⅰ-3 was a white solid (yield 71%). 1 H NMR (400MHz, CDCl 3 )δ7.84–7.82(m,2H),7.75–7.73(m,2H),4.94–4.91(m,1H),2.02–1.92(m,4H),1.80–1.74(m,2H),1.64– 1.58(m,2H). 13 CNMR (101MHz, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com