Method for preparing tetrasubstituted furan compound based on carbon-hydrogen bond activation
A compound and tetra-substituted technology, applied in the field of preparing tetra-substituted furan compounds, can solve the problems of seriousness, pollution and high price, and achieve the effects of realizing reaction conditions, easy reaction conditions and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
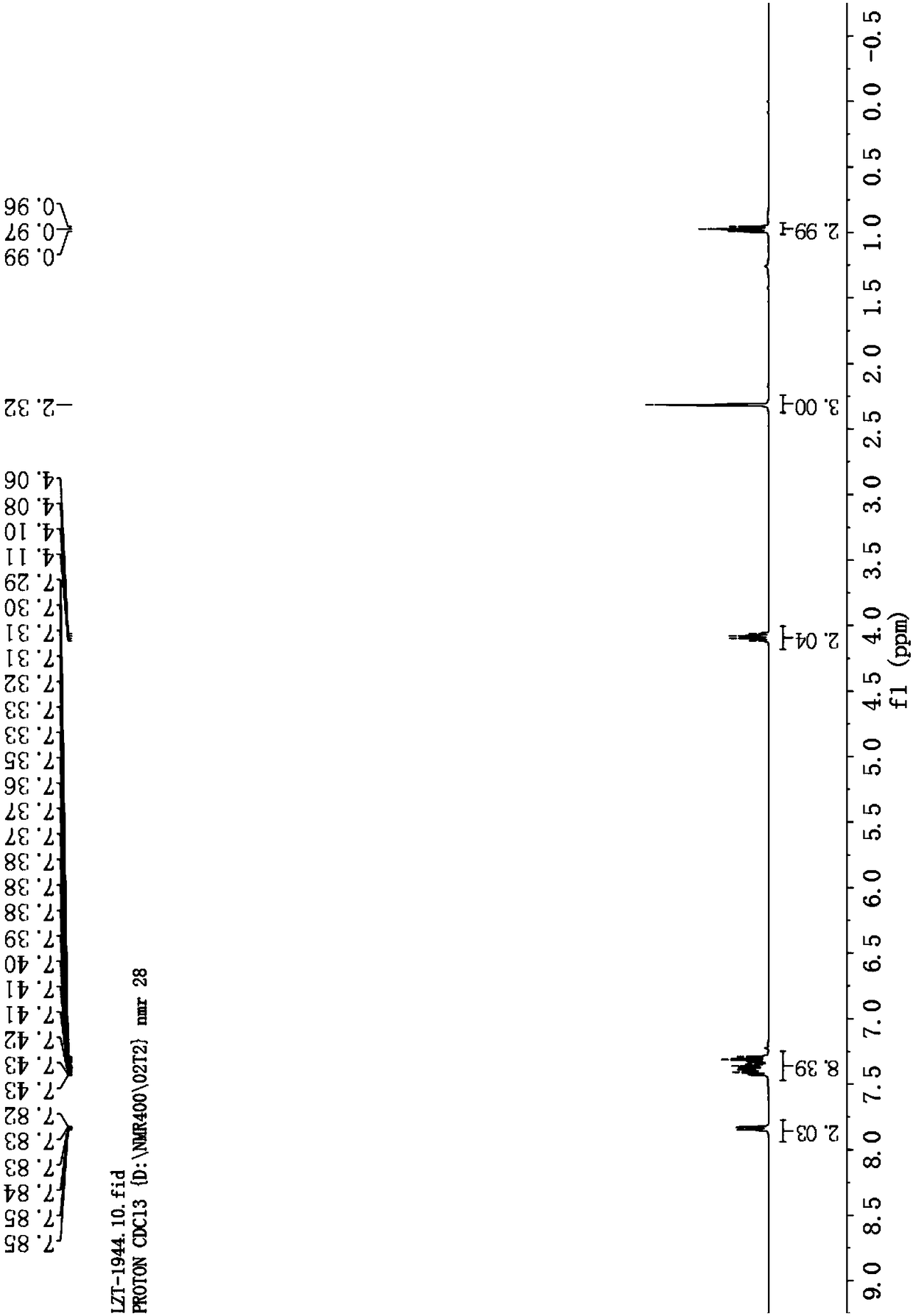
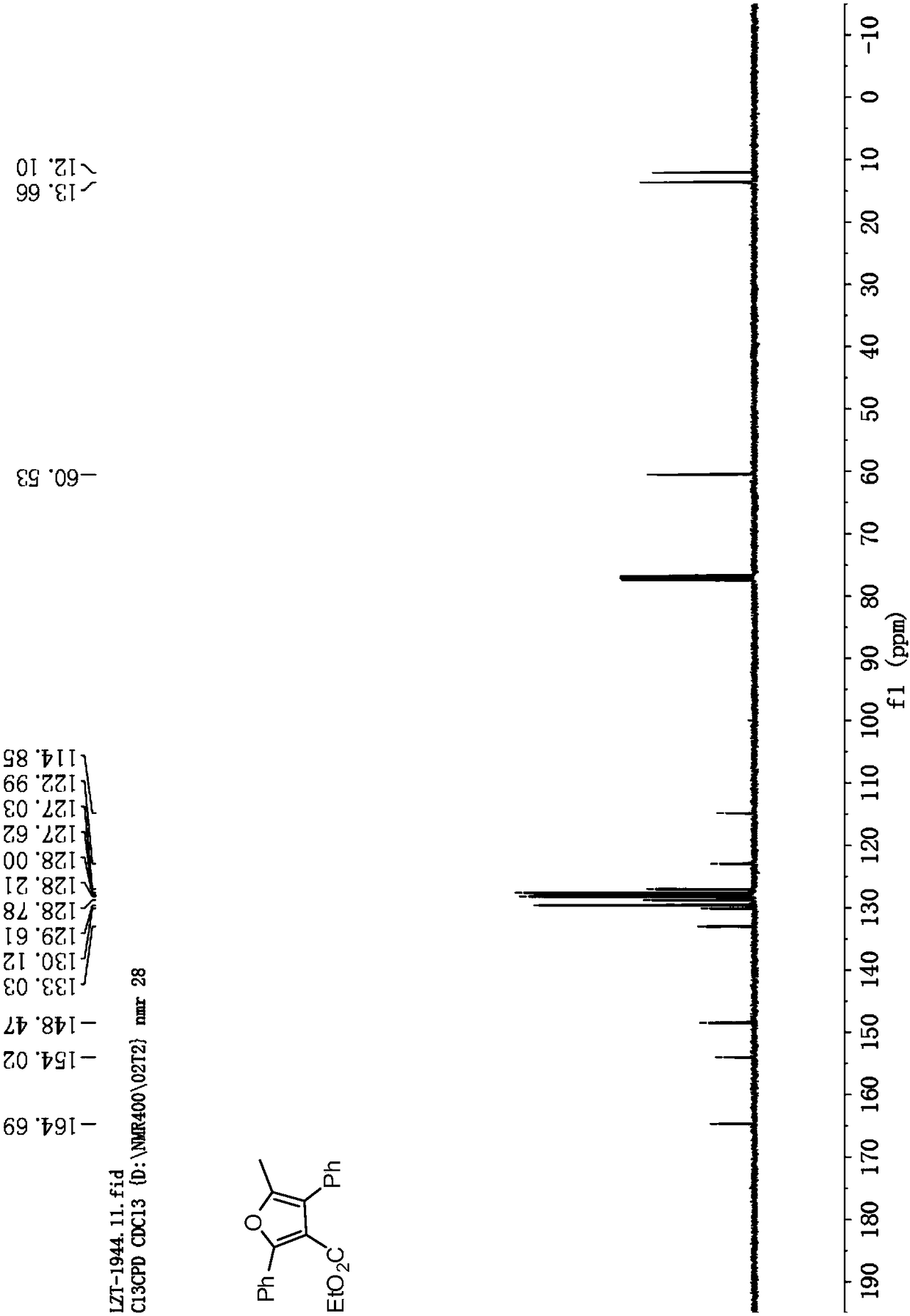
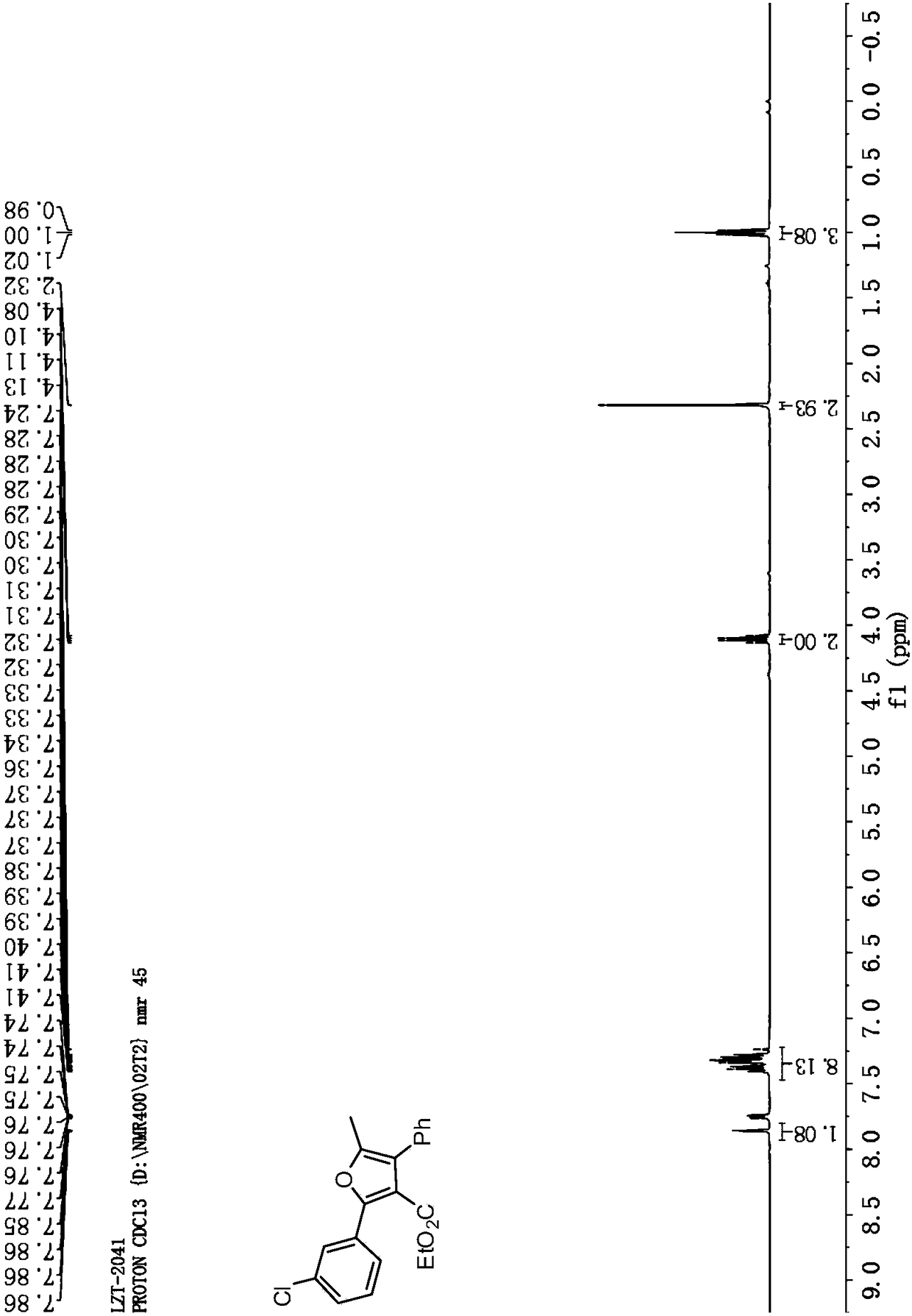
[0058] Cu(OTf) 2 Complexation with L-1 is used as a catalyst to catalyze the reaction to generate tetrasubstituted furan addition product 5-methyl-2,4-diphenylfuran-3-carboxylic acid ethyl ester Ⅰ-1.
[0059] Add metal precursor Cu(OTf) to the reaction flask 2 (0.01mmol, 5mol%) and o-phenanthroline monohydrate ligand L-1 (0.011mmol, 5.5mol%), added 1.0mL of anhydrous dichloromethane under nitrogen protection, and stirred at room temperature for 1 hour. Then phenylpropyne Ⅲ-1 (0.44mmol, 2.2equiv), ethyl benzoyl acetate Ⅱ-1 (0.2mmol, 1.0equiv), Et 3 N (0.24mmol, 1.2equiv) and Ag 2 CO 3 (0.24mmol, 1.2equiv) was dissolved in 2.0mL of anhydrous dichloromethane, and then the solution was added to the above-mentioned stirred catalyst solution under nitrogen protection, and the reaction was stirred at 40°C for 24h; then DBU (0.24mmol, 1.2 equiv), continue to reflux at 40°C, and monitor the reaction by TLC. After the reaction was completed, it was concentrated under reduced pressu...
Embodiment 2
[0064] L-2 acts as a ligand to react to produce ethyl 5-methyl-2,4-diphenylfuran-3-carboxylate Ⅰ-1.
[0065] The ligand L-1 in Example 1 is replaced by the ligand L-2, and the rest are the same as in Example 1. The reaction gave compound I-1 in 41% yield.
[0066] The structural formula of L-2 is as follows:
[0067]
Embodiment 3
[0069] L-3 reacted as a ligand to generate the product ethyl 5-methyl-2,4-diphenylfuran-3-carboxylate Ⅰ-1.
[0070] The ligand L-1 in Example 1 is replaced by the ligand L-3, and the rest are the same as in Example 1. The reaction gave compound I-1 in 64% yield.
[0071] The structural formula of L-3 is as follows:
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com