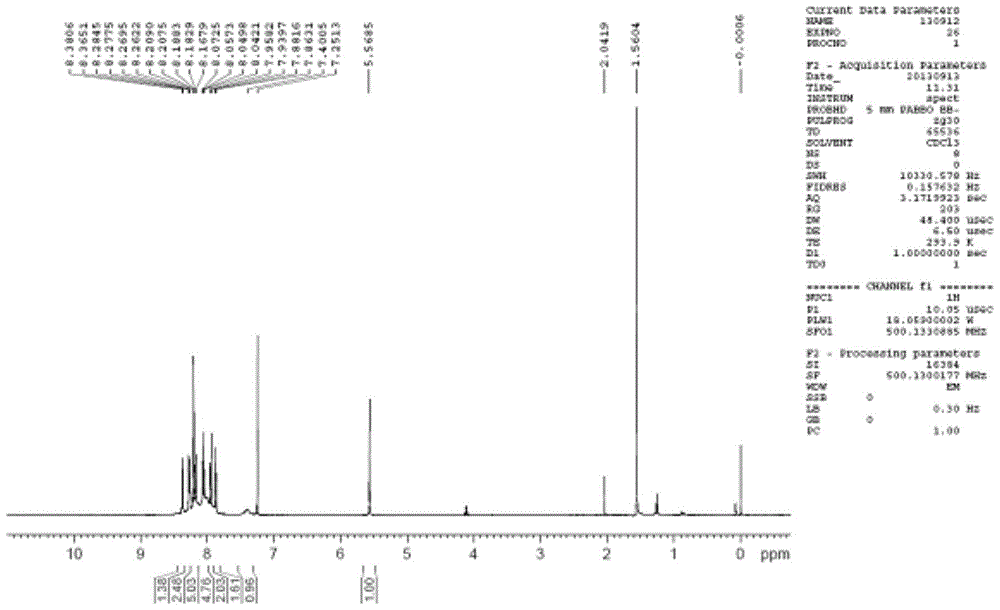
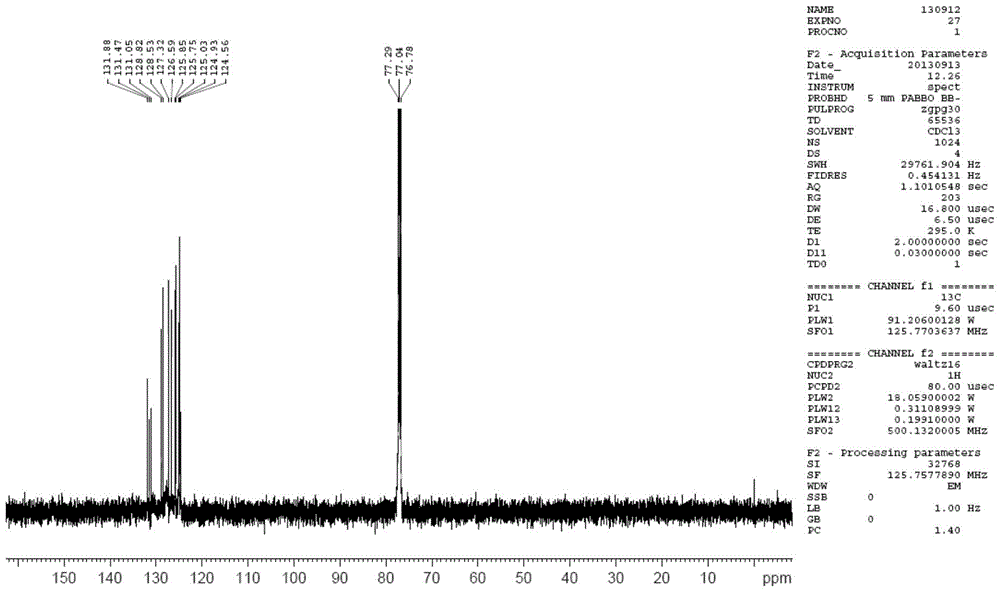
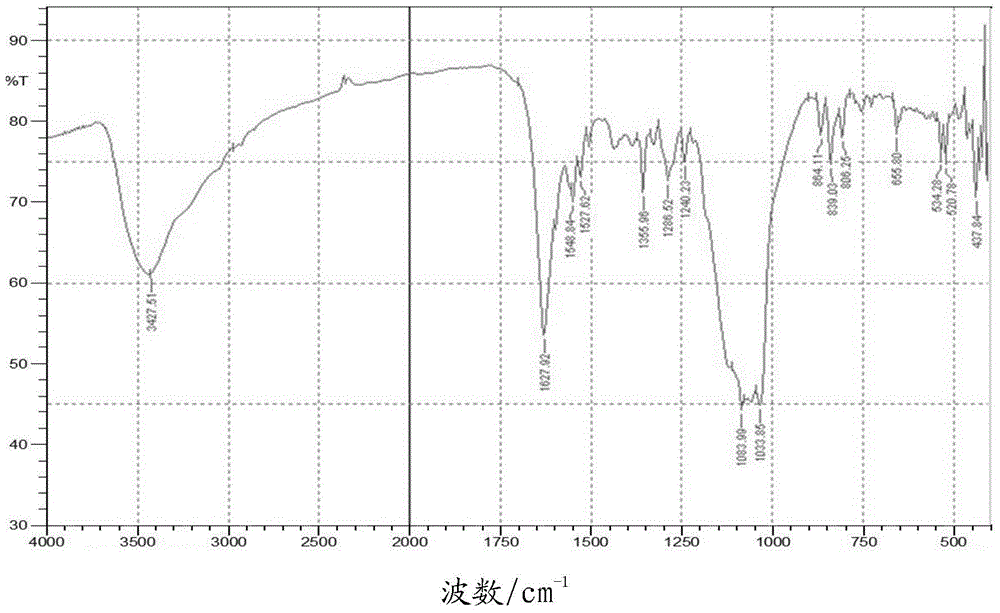
A kind of bipyrene and its preparation method and use
A technology of bipyrene and hydroxypyrene, which is applied in the field of synthesis of photoresist materials, can solve the problems of harsh reaction conditions, low reaction yield, and restrictions on the wide application of bipyrene phenolic compounds, and achieves mild reaction conditions and excellent preparation methods. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 bipyrenol
[0030] In a 250mL three-necked flask, add 4.36g 1-hydroxypyrene (20mmol), 11.24g 1-bromopyrene (40mmol) and 4.48g potassium tert-butoxide (40mmol), then add 50mL o-xylene, mechanically stir, and add to the reaction The nitrogen gas was continuously passed through the vessel for 10 minutes to obtain a mixed solution. Raise the temperature to 40-60°C, blow nitrogen and stir for 10 minutes to remove the oxygen in the reaction flask, add 4.5mgPd(OAc) 2 (0.02mmol) and 14.3mg of bis(1-adamantyl)butylphosphine (0.04mmol), continue to heat up to 80-100°C, and react for 2-6h. TLC followed the reaction until no 1-hydroxypyrene remained. Add 50mL of tetrahydrofuran to the reaction solution, filter, and concentrate the filtrate under reduced pressure to remove the organic solvent to obtain 9.7g of brown solid, add 48.5g of toluene and absolute ethanol mixture (the mass ratio of toluene and absolute ethanol is 2:1), Heat to dissolve, co...
Embodiment 2
[0032] The preparation of embodiment 2 bipyrenol
[0033] In a 250mL three-necked flask, add 4.36g 1-hydroxypyrene (20mmol), 11.24g 1-bromopyrene (40mmol) and 3.84g sodium tert-butoxide (40mmol), then add 50mL o-xylene, mechanically stir, and add to the reaction Continue to pass nitrogen in the container for 10 minutes to obtain a mixed solution, raise the temperature to 40-60°C, pass nitrogen and stir for 10 minutes to remove oxygen in the reaction flask, add 4.5mgPd(OAc) 2 (0.02mmol) and 14.3mg of bis(1-adamantyl)butylphosphine (0.04mmol), continue to heat up to 80-100°C, and react for 2-6h. TLC followed the reaction until no 1-hydroxypyrene remained. Add 50mL of tetrahydrofuran to the reaction solution, filter, and concentrate the filtrate under reduced pressure to remove the organic solvent to obtain 9.3g of brown solid, add 46.5g of toluene and absolute ethanol mixture (the mass ratio of toluene and absolute ethanol is 2:1), Heat to dissolve, cool down naturally, a ligh...
Embodiment 3
[0035] The preparation of embodiment 3 bipyrenol
[0036] In a 250mL three-necked flask, add 4.36g 1-hydroxypyrene (20mmol), 11.24g 1-bromopyrene (40mmol) and 4.48g potassium tert-butoxide (40mmol), then add 50mL o-xylene, mechanically stir, and add to the reaction Continue to pass nitrogen in the container for 10 minutes to obtain a mixed solution, raise the temperature to 40-60°C, pass nitrogen and stir for 10 minutes to remove oxygen in the reaction flask, add 4.5mgPd(OAc) 2 (0.02mmol) and 8.1mg tri-tert-butylphosphine (0.04mmol), continue to heat up to 80-100°C, and react for 2-6h. TLC followed the reaction until no 1-hydroxypyrene remained. Add 50mL of tetrahydrofuran to the reaction solution, filter, and concentrate the filtrate under reduced pressure to remove the organic solvent to obtain 8.9g of brown solid, add 44.5g of toluene and absolute ethanol mixture (the mass ratio of toluene and absolute ethanol is 2:1), Heat to dissolve, cool down naturally, a light yellow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com