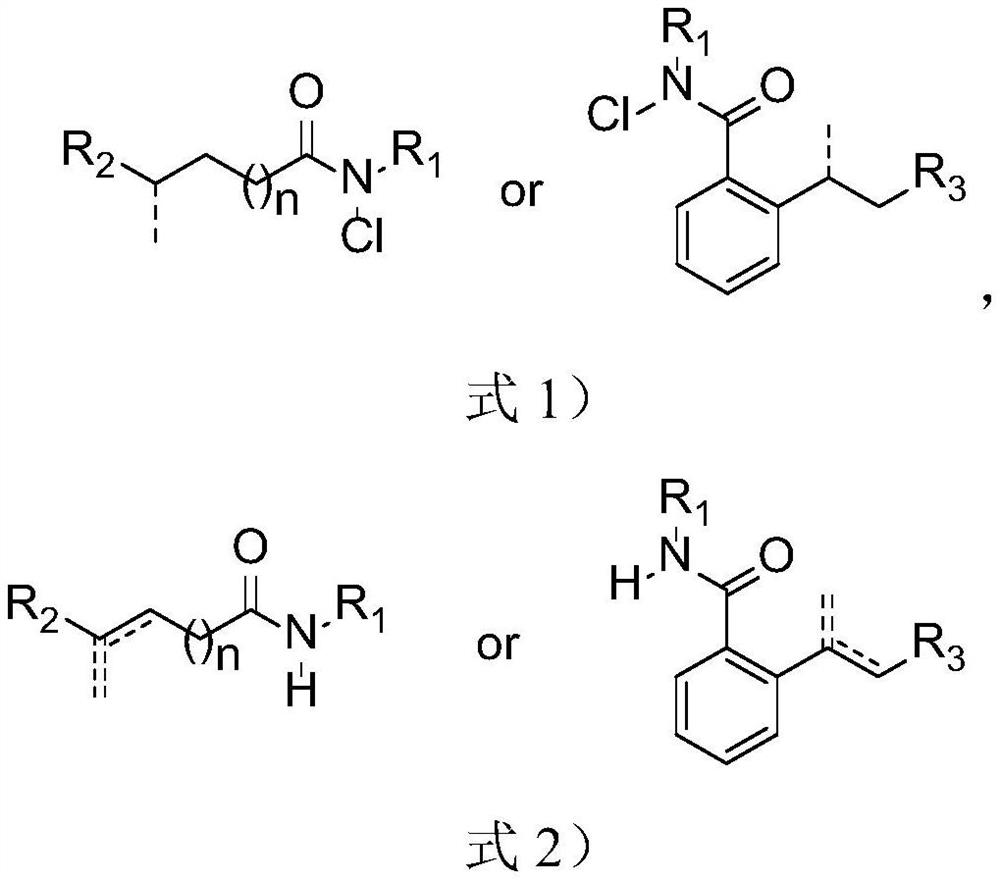
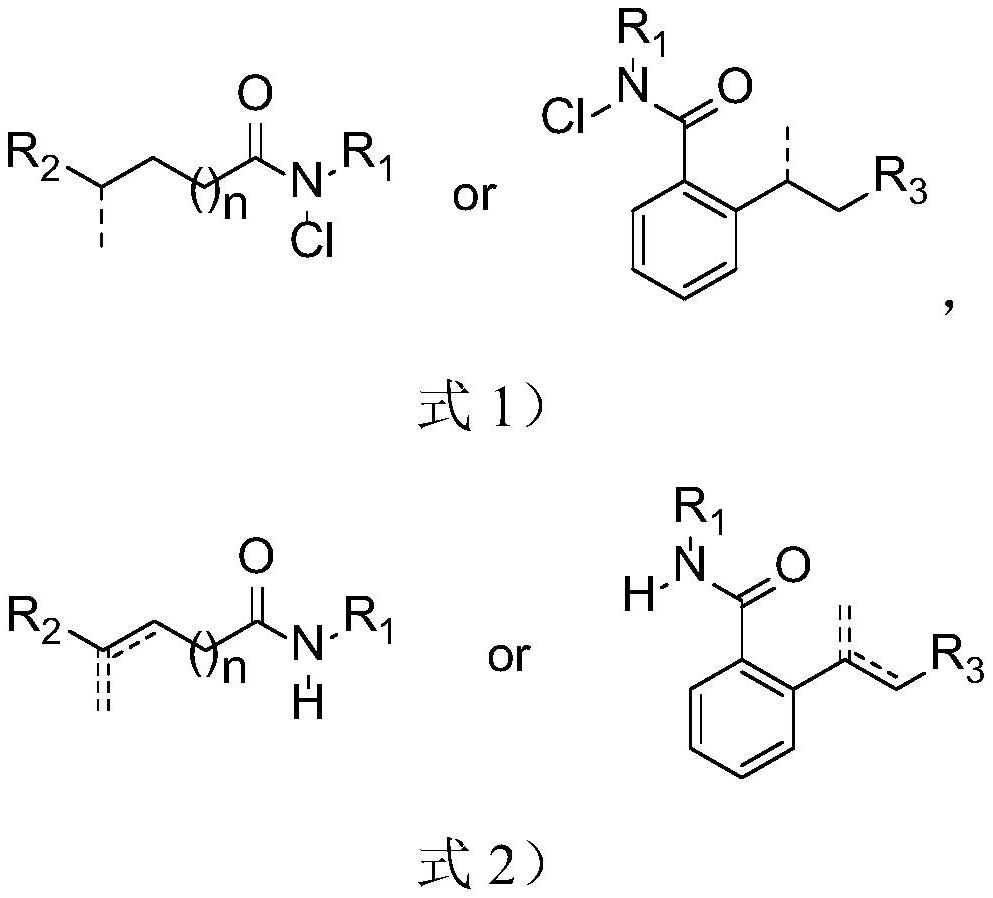
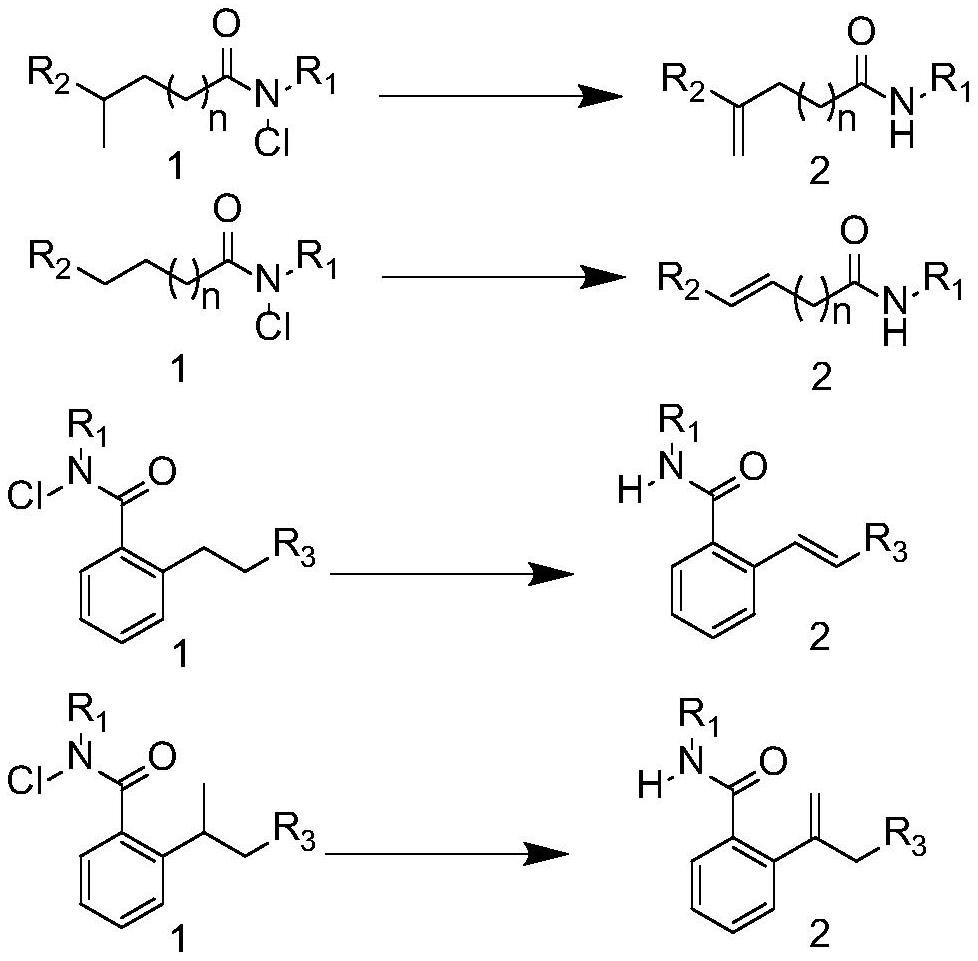
Method for synthesizing olefin through selective desaturation of inert carbon-carbon bonds
A carbon-carbon bond, selective technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of harsh reaction conditions, low selectivity and efficiency, and limited substrate range, and achieve reaction efficiency. High, high application value, good effect of regional selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Add 0.2mmol chloroamide 1a, [RuCl 2 (p-cymene)] 2 (10mol%, 12.8mg), ligand L1 (20mol%), KOAc (0.4mmol, 40mg), H 2 O (1mmol, 18mg), was added with 1,2-dichloroethane (4mL) for lyophilization and deoxygenation, under nitrogen protection. Seal the tube, and react under the irradiation of 38W 460nm blue light for 48 hours unless otherwise specified. After the reaction, the reaction mixture was concentrated to obtain a crude product, which was purified by column chromatography to obtain olefinic compound 2a. The yield is 58% yield, E / Z≧20 / 1, and the E / Z ratio is obtained from the integral ratio of the isomer olefin hydrogen atom characteristic signal peaks in the H NMR spectrum of the crude product.
[0033] 1 H NMR (400MHz, CDCl 3 )δ7.38(d, J=7.4Hz, 2H), 7.32(t, J=7.4Hz, 2H), 7.23(d, J=7.2Hz, 1H), 6.50(d, J=15.9Hz, 1H) ,6.29(dt,J=15.8,7.3Hz,1H), 5.42(s,1H),3.07(d,J=8.4Hz,2H),1.35(s,9H).
[0034] 13 C NMR (101MHz, CDCl 3 )δ169.9, 136.7, 134.1, 128.6, 1...
Embodiment 2
[0037] Example 2: On the basis of Example 1, replace L1 (20 mol%) with L2 (20 mol%), and the rest are the same as Example 1 to synthesize compound 2a with a yield of 26%, E / Z≧20 / 1.
Embodiment 3
[0038] Example 3: On the basis of Example 1, replace L1 (20 mol%) with L3 (20 mol%), and the rest are the same as Example 1 to synthesize compound 2a with a yield of 49%, E / Z≧20 / 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com