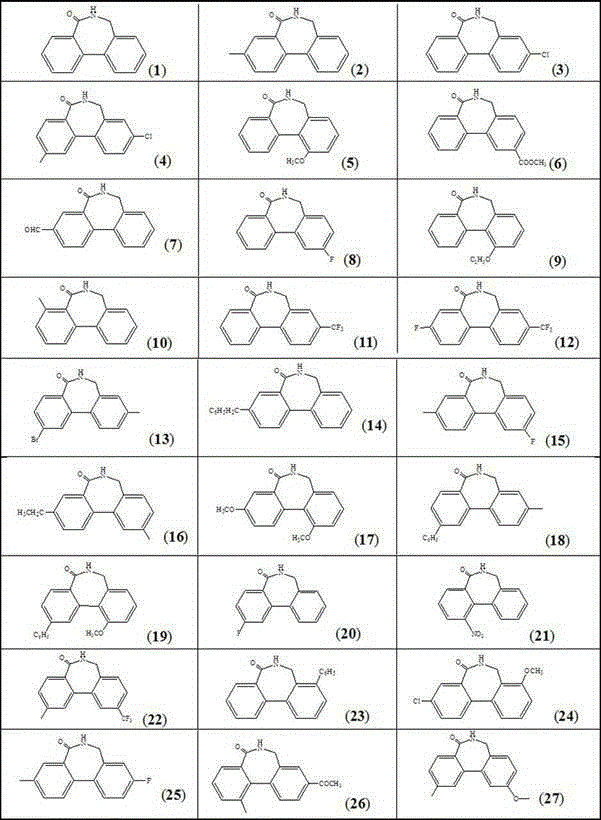
Diaryl-azepin-5-one compound synthesis method
A ketone compound and a synthesis method technology, applied in the field of organic synthesis, can solve the problems of cumbersome preparation, large environmental pollution, hard-to-obtain species and the like, and achieve the effects of economical and efficient reaction, low environmental pollution, and wide range of cheapness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of biaryl azepin-5-ones compound (1): Under nitrogen protection, add 1.0mmol o-bromobenzyl alcohol, 2.0mmol benzonitrile, 0.02mmol RuH 2 (PPh 3 ) 4 , 0.03mmol palladium acetate, 0.05mmol 1,3-bis(2,6-diisopropylphenyl)imidazole chloride, 2.0mmol sodium hydride, and 5ml toluene, replace the reaction tube with nitrogen for 3 times, and then stir the Heated to 110°C with an oil bath, and the reaction was refluxed for 24 hours. Remove the oil bath and lower to room temperature; add 3ml of water to the reaction solution, extract three times with 5ml of dichloromethane, combine the organic phases and wash with anhydrous MgSO 4 Dry for 30 minutes and filter; the filtrate is concentrated with a rotary evaporator, and the concentrated solid is recrystallized with dichloromethane as a solvent to obtain pure product 1 , 85% yield. The nuclear magnetic analysis data of this product are as follows: 1 H NMR. (400 MHz, CDCl 3 ): δ 8.01 (d, 1H), 7.69–7.56 (m, 3H), 7....
Embodiment 2
[0038] Preparation of biaryl azepin-5-ones compound (3): Under nitrogen protection, add 1.0mmol benzyl alcohol, 1.2mmol 2-bromo-5-chlorobenzonitrile, 0.1mmol RuH 2 (CO)(PPh 3 ) 3 , 0.1 mmol palladium chloride, 0.15 mmol 1,3-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazolium tetrafluoroborate, 3.0 mmol sodium tert-butoxide, and 5 ml benzene, The reaction tube was replaced with nitrogen for 3 times, then heated to 100° C. in an oil bath under magnetic stirring, and the reaction was refluxed for 6 hours. Remove the oil bath and lower to room temperature; add 3ml of water to the reaction solution, extract three times with 5ml of dichloromethane, combine the organic phases and wash with anhydrous MgSO 4 Dry for 30 minutes and filter; the filtrate is concentrated with a rotary evaporator, and the concentrated solid is recrystallized with dichloromethane as a solvent to obtain pure product 2 , yield 82%. The nuclear magnetic analysis data of this product are as follows: 1 H NMR...
Embodiment 3
[0040] Preparation of biaryl azepin-5-ones (6): Under the protection of nitrogen, add 1.0mmol benzyl alcohol, 1.1mmol 2-bromo-4-methoxybenzonitrile, 0.05mmol RuH 2 (CO)(PPh 3 ) 3 ,, 0.06 mmol of palladium acetate, 0.1 mmol of 1,3-bis(2,6-diisopropylphenyl) imidazole bromide, 6.0 mmol of potassium tert-butoxide, and 5 ml of dioxane, and replace reaction tube 3 with nitrogen gas times, and then heated to 110°C with an oil bath under magnetic stirring, and the reaction was refluxed for 10 hours. Remove the oil bath and lower to room temperature; add 3ml of water to the reaction solution, extract three times with 5ml of dichloromethane, combine the organic phases and wash with anhydrous MgSO 4 Dry for 30 minutes and filter; the filtrate is concentrated with a rotary evaporator, and the concentrated solid is recrystallized with dichloromethane as a solvent to obtain pure product 6 , 80% yield. The nuclear magnetic analysis data of this product are as follows: 1 H NMR. (400 M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com