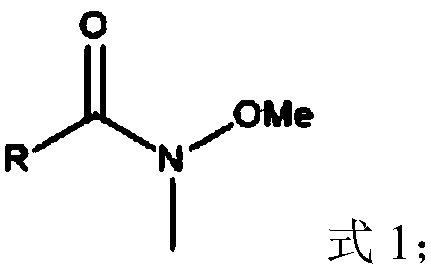
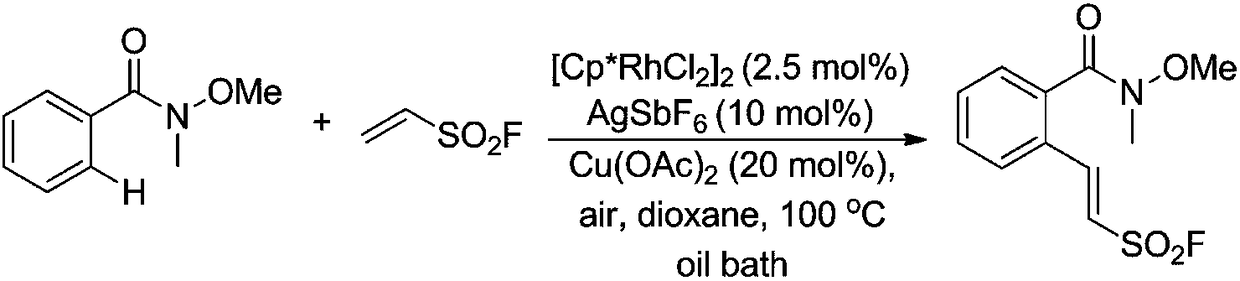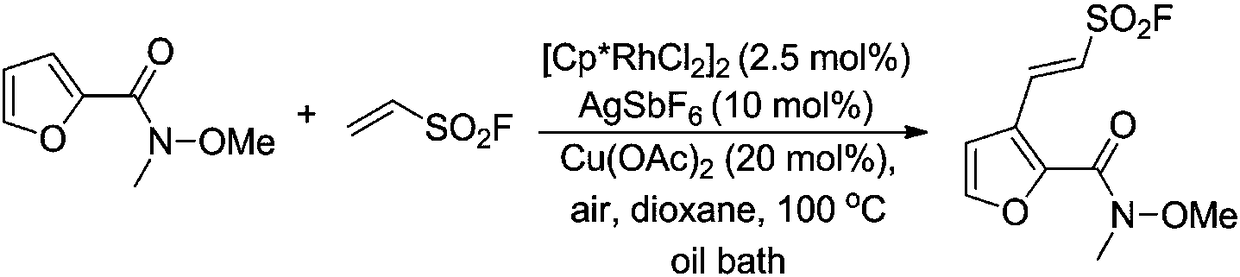Method for preparing sulfonyl fluoride compound by activating carbon-hydrogen bond
A compound, sulfonyl fluoride technology, applied in the field of medicinal chemistry, can solve problems such as poor tolerance of functional groups, limited application, unstable raw materials, etc., and achieve the effect of good functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] To a 25 mL reaction tube was added N-methyl N-methylbenzamide (0.5 mmol), vinylsulfonyl fluoride (0.75 mmol), [Cp*RhCl 2 ] 2 (2.5mol%), AgSbF 6 (10mol%), Cu(OAc) 2 (20mol%), 1,4-dioxane (6mL), stirred and reacted in an oil bath at 100°C for 15h, the reaction solution was distilled under reduced pressure to recover 1,4-dioxane and vinylsulfonyl fluoride, and the residue was used Silica gel column chromatography purification (eluent is petroleum ether: ethyl acetate = 5:1 (v / v)), that is, yellow liquid (E)-2-(2-(methoxy(methyl)aminomethyl) Acyl)phenyl)vinylsulfonyl fluoride (123 mg, 90% yield). 1 HNMR (500MHz, CDCl 3 )δ7.88(d, J=15.5Hz, 1H), 7.62(d, J=7.6Hz, 1H), 7.56-7.47(m, 3H), 6.87(d, J=15.5Hz, 1H), 3.42( s,3H),3.36(s,3H). 19 FNMR (471MHz, CDCl 3 )δ61.8(s,1F). 13 CNMR (126MHz, CDCl 3)δ168.5(s), 146.0(d, J=2.0Hz), 136.6(s), 131.8(s), 130.1(s), 128.6(s), 128.0(s), 127.4(s), 120.2( d,J=27.7Hz),61.3(s),32.7(s).HRMSESI(m / z):[M+Na] + calcdforC 11 h ...
Embodiment 2
[0035]
[0036] To a 25 mL reaction tube was added N-methyl-N-methoxy-2-furancarboxamide (0.5 mmol), vinylsulfonyl fluoride (0.75 mmol), [Cp*RhCl 2 ] 2 (2.5mol%), AgSbF 6 (10mol%), Cu(OAc) 2 (20mol%), 1,4-dioxane (6mL), stirred and reacted in an oil bath at 100°C for 15h, the reaction solution was distilled under reduced pressure to recover 1,4-dioxane and vinylsulfonyl fluoride, and the residue was used Silica gel column chromatography purification (eluent is petroleum ether: ethyl acetate = 3:1 (v / v)), to obtain white solid (E)-2-(2-(methoxy(methyl)aminomethyl) Acyl)furyl)vinylsulfonyl fluoride (110.5 mg, 84% yield). 1 HNMR (500MHz, CDCl 3 )δ8.35(d, J=15.6Hz, 1H), 7.53(d, J=1.7Hz, 1H), 6.75(d, J=15.6Hz, 1H), 6.70(d, J=1.7Hz, 1H) ,3.82(s,3H),3.34(s,3H). 19 FNMR (471MHz, CDCl 3 )δ62.2(s,1F). 13 CNMR (126MHz, CDCl 3 )δ159.3(s), 146.9(s), 144.6(s), 139.5(s), 124.3(s), 120.4(d, J=28.7Hz), 109.2(s), 62.2(s), 33.7( s).Mp95-97℃.HRMSESI(m / z):[M+Na] + calcdforC 9 h 10 ...
Embodiment 3
[0038]
[0039] Add N,4-dimethoxy-N-methylbenzamide (0.5 mmol), vinylsulfonyl fluoride (0.75 mmol), [Cp*RhCl 2 ] 2 (2.5mol%), AgSbF 6 (0.5mmol), Cu(OAc) 2 (20mol%), 1,4-dioxane (6mL), stirred and reacted in an oil bath at 100°C for 15h, the reaction solution was distilled under reduced pressure to recover 1,4-dioxane and vinylsulfonyl fluoride, and the residue was used Purified by silica gel column chromatography (eluent is petroleum ether: ethyl acetate = 3:1 (v / v)) to obtain a white solid (6-methoxy-3-oxa-1,3-dihydroiso Benzofuran)methanesulfonyl fluoride (98.9 mg, 76% yield). 1 HNMR (500MHz, DMSO-d6) δ7.79(d, J=8.5Hz, 1H), 7.44(s, 1H), 7.18(d, J=8.5Hz, 1H), 6.03(d, J=8.5Hz, 1H), 5.02(dd, J=15.1, 9.6Hz, 1H), 4.61(dd, J=15.2, 9.4Hz, 1H), 3.89(s, 3H). 19 FNMR (471MHz, DMSO) δ61.0 (d, J=9.8Hz, 1F). 13 CNMR (126MHz, DMSO-d6) δ169.0(s), 165.0(s), 149.8(s), 127.2(s), 118.0(s), 117.7(s), 107.9(s), 74.3(s), 56.5(s), 53.9(d, J=13.8Hz).Mp175-177℃.HRMSESI(m / z):[M+Na] + calcd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com