A method for rapid preparation of benzylselenide compounds based on selenium-directed carbon-hydrogen bond boronation
A technology for carbon-hydrogen bond boron and selenium compounds is applied in the field of rapid preparation of benzyl selenium compounds based on selenium-directed carbon-hydrogen bond boronation, and can solve the problem of great harm to the human body and the environment, few types of selenium-containing compounds, difficult to obtain raw materials and prices, etc. problem, to achieve good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
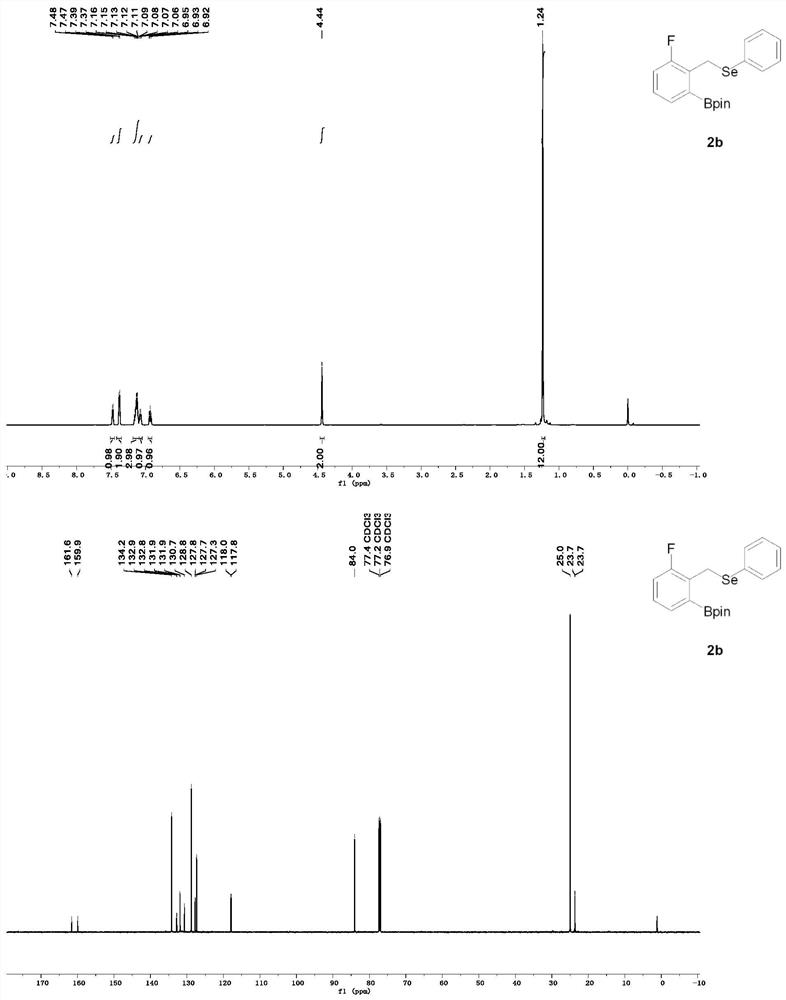
[0046] Synthesis of 2-(3-fluoro-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane.
[0047] Weigh (1,5-cyclooctadiene) iridium (I) dichloride dimer (2.7 mg, 0.004 mmol) tricyclohexylphosphine (2.2 mg, 0.008 mmol) into a Shrek tube, under inert gas protection At 80 ° C for 20 min, add bis-boronic acid pinacol ester (25.4 mg, 0.1 mmol), add (2-fluorobenzyl) (phenyl) selenium (53.2 mg, 0.2 mmol) and pinacol borane (100 μL, 6.8 mmol) The reaction was stirred at 80° C. for 24 h. The resulting reactant was purified by silica gel column (petroleum ether / ethyl acetate) to give 2-(3-fluoro-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl -1,3,2-Dioxaborane (62 mg) in 80% yield.
[0048] The structural formula of 2-(3-fluoro-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane is as follows:
[0049]
[0050] NMR of the product 2-(3-fluoro-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane Hydrogen and carbon NMR ...
Embodiment 2
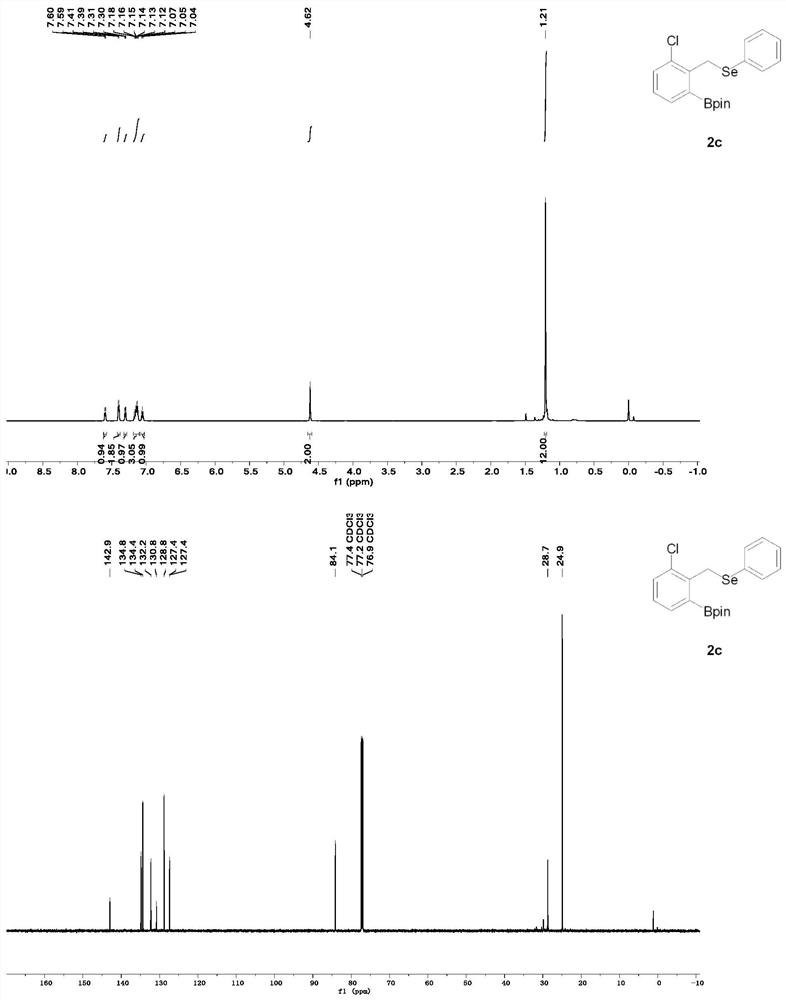
[0052] Synthesis of 2-(3-chloro-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane.
[0053] Weigh (1,5-cyclooctadiene) iridium (I) dichloride dimer (2.7 mg, 0.004 mmol) tricyclohexylphosphine (2.2 mg, 0.008 mmol) into a Shrek tube, under inert gas protection At 80° C. for 20 min, bis-boronic acid pinacol ester (25.4 mg, 0.1 mmol) was added, (2-chlorobenzyl) (phenyl) selenium (56.4 mg, 0.2 mmol) and pinacol borane were added. (100 μL, 6.8 mmol) The reaction was stirred at 80° C. for 24 h. The resulting reactant was purified by silica gel column (petroleum ether / ethyl acetate) to obtain 2-(3-chloro-2-(((phenylselenoyl)methyl)phenyl)-4,4,5,5-tetramethyl -1,3,2-dioxaborane (62 mg) in 76% yield.
[0054] The structural formula of 2-(3-chloro-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane is as follows:
[0055]
[0056] Hydrogen NMR of 2-(3-Chloro-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane Spectrosco...
Embodiment 3
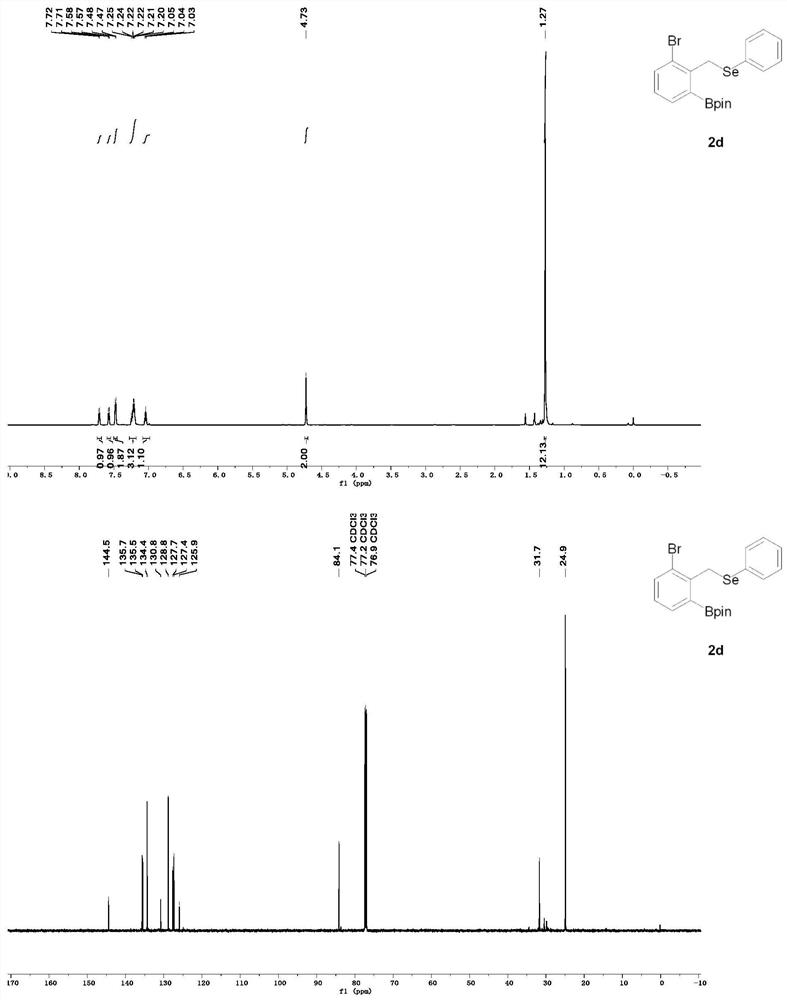
[0058] Synthesis of 2-(3-bromo-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane.
[0059] Weigh (1,5-cyclooctadiene) iridium (I) dichloride dimer (2.7 mg, 0.004 mmol) tricyclohexylphosphine (2.2 mg, 0.008 mmol) into a Shrek tube, under inert gas protection At 80° C. for 20 min, bis-boronic acid pinacol ester (25.4 mg, 0.1 mmol) was added, (2-bromobenzyl) (phenyl) selenium (65.2 mg, 0.2 mmol) and pinacol borane were added. (100 μL, 6.8 mmol) The reaction was stirred at 80° C. for 24 h. The resulting reactant was purified by silica gel column (petroleum ether / ethyl acetate) to give 2-(3-bromo-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl -1,3,2-dioxaborane (59 mg) in 66% yield.
[0060] The structural formula of 2-(3-bromo-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane is as follows:
[0061]
[0062] Hydrogen NMR of 2-(3-Bromo-2-(((phenylselenyl)methyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane Spectroscopy and C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com