Phenanthroimidazole-isoquinoline, derivative, preparation method and application thereof
A technology of imidazoles and compounds, applied in the field of catalytic synthesis of fine chemical products, achieving the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
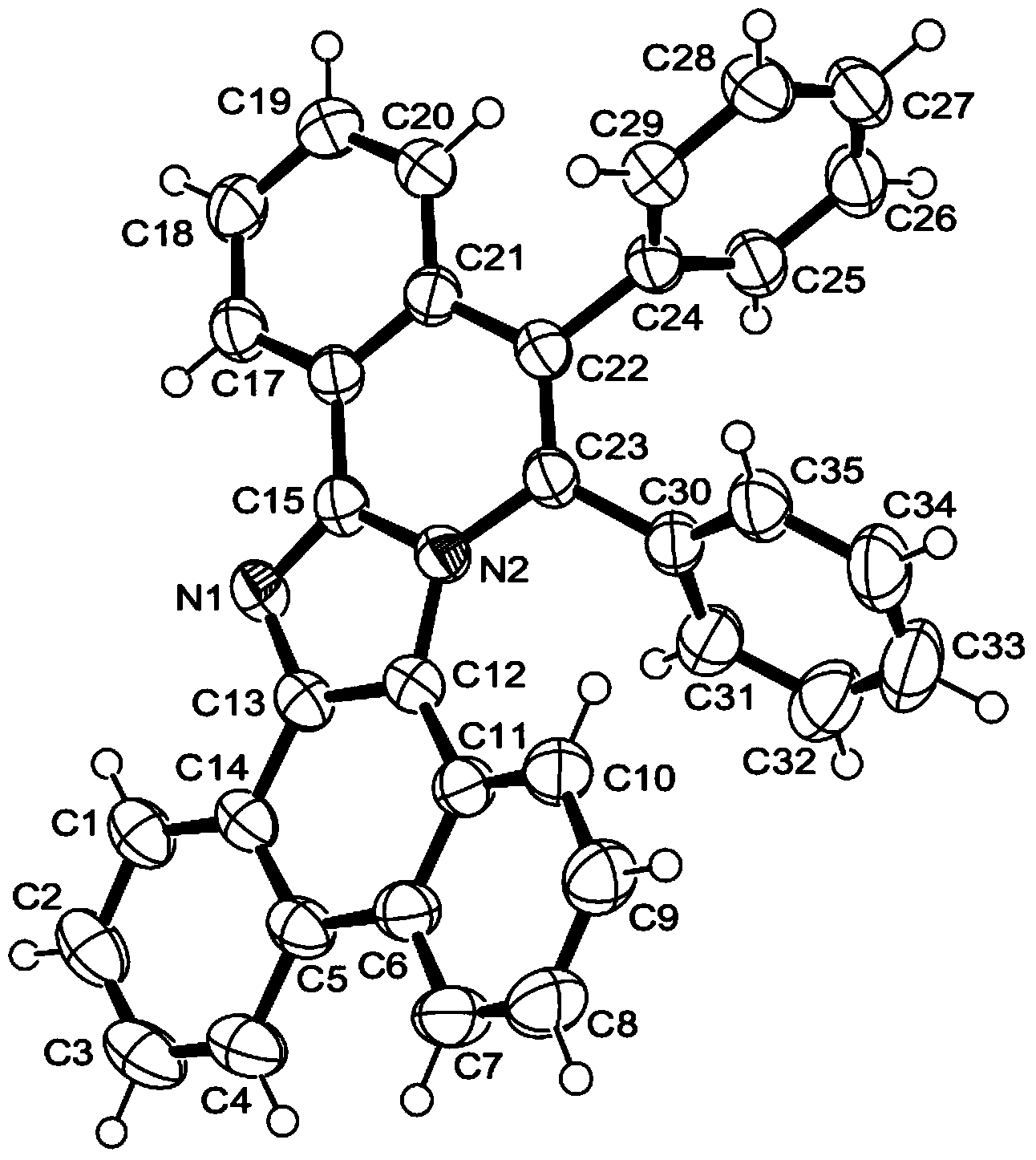
[0052] Sequentially weigh 147.2mg of compound 2-phenylphenanthroimidazole represented by formula II (0.5mmol), 124.8mg of compound toluene shown in formula III (0.7mmol), 7.7mg of catalyst [Cp*RhCl 2 ] 2 (2.5% of the molar dosage of the compound represented by formula II) and 220.0 mg of oxidant copper acetate monohydrate (1.1 mmol) were placed in a 25 mL sealed tube containing a magnetic stirrer, and 5 mL of acetone was added. After sealing the sealed tube, put it into an oil bath at 120° C. and stir for 12 hours to perform a cyclization reaction based on carbon-hydrogen bond activation. After the reaction was completed, column separation was performed using petroleum ether-acetone as the eluent to obtain 194.5 mg of a light yellow solid, and the isolated yield of the target product 5,6-diphenylphenanthroimidazoisoquinoline was 83%.
[0053] figure 1 It is the crystal structure diagram of the product obtained in this example, and the structure of the compound can be seen fr...
Embodiment 2
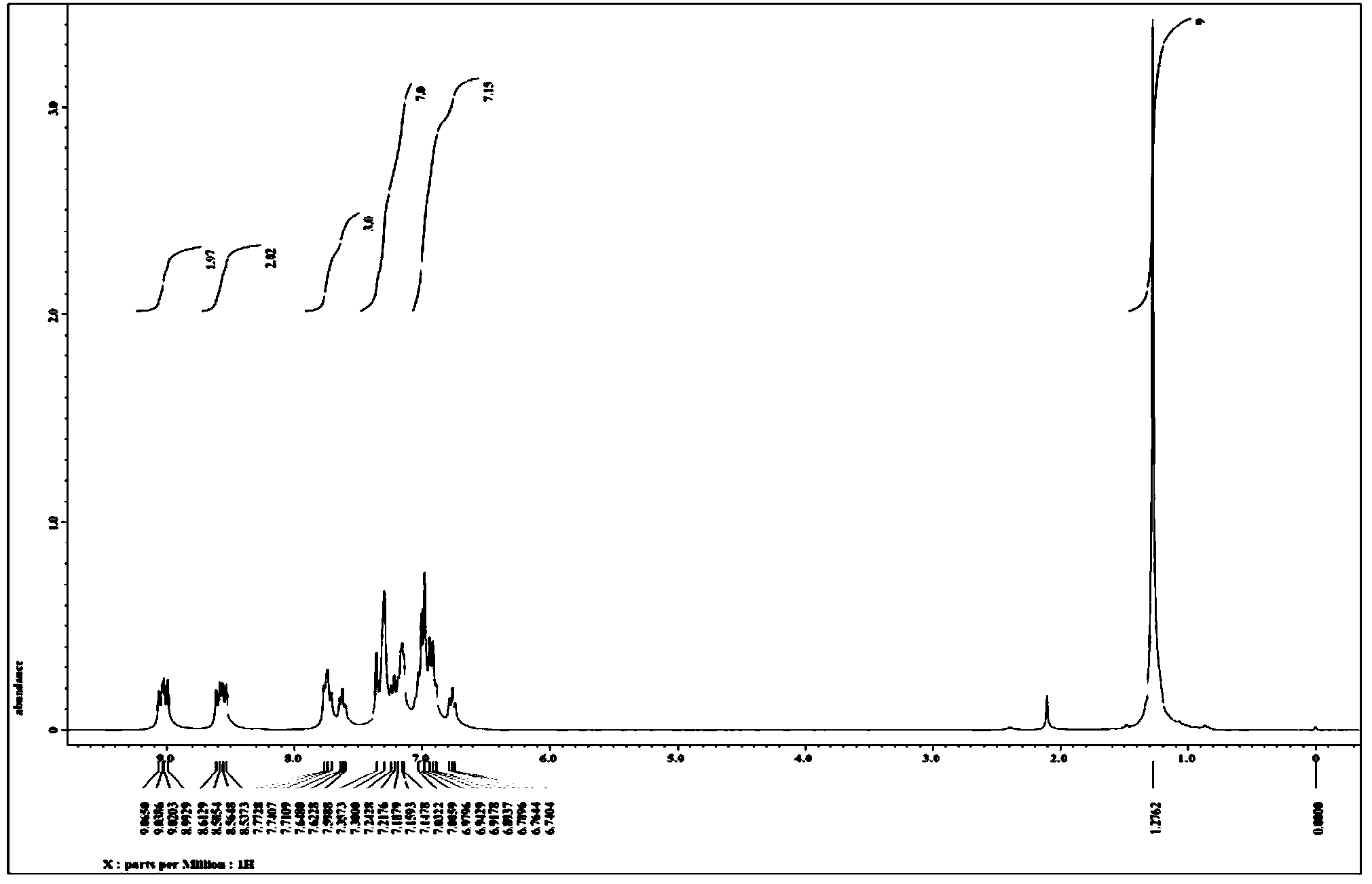
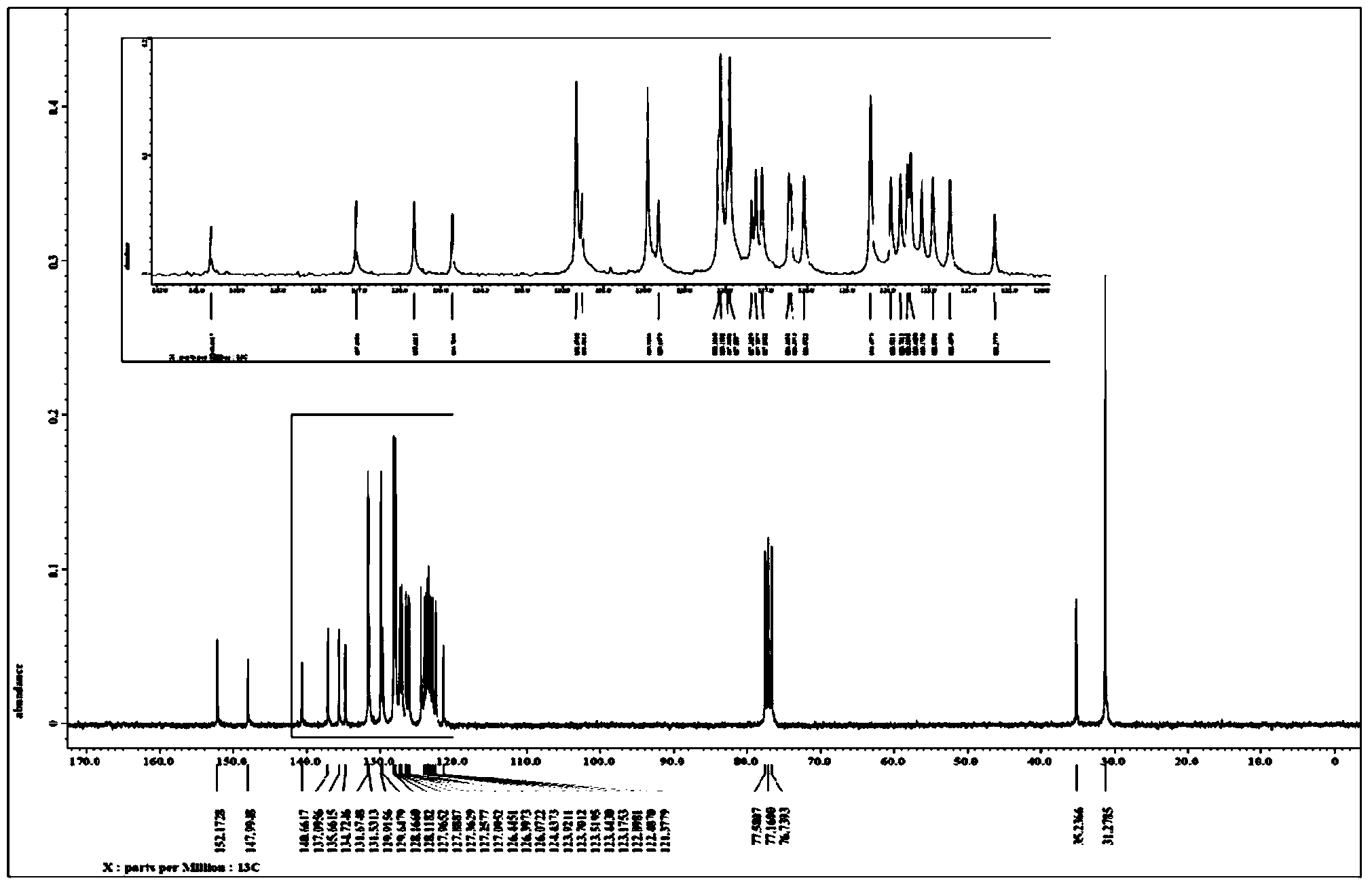
[0055] Weigh in turn 175.2mg of compound 2-(p-tert-butylphenyl)phenanthroimidazole (0.5mmol), 124.8mg of tolanylacetylene (0.7mmol), 7.7mg of [Cp*RhCl 2 ] 2 (2.5% of the molar dosage of the compound represented by formula II) and 220.0 mg of copper acetate monohydrate (1.1 mmol) were placed in a 25 mL sealed tube containing a magnetic stirrer, and 5 mL of acetone was added. After sealing the sealed tube, put it into an oil bath at 120° C. and stir for 12 hours to perform a cyclization reaction based on carbon-hydrogen bond activation. After the reaction, use petroleum ether-acetone as the eluent to carry out column separation to obtain 242.7 mg of off-white solid, and the isolated yield of the target product 3-tert-butyl-5,6-diphenylphenanthroimidazoisoquinoline is 92%.
[0056] figure 2 and image 3 The proton nuclear magnetic resonance spectrum and carbon spectrum of the product prepared in this embodiment are respectively, and the structure of the compound can be seen ...
Embodiment 3
[0058] Sequentially weigh 162.2mg of compound 2-(p-methoxyphenyl)phenanthroimidazole (0.5mmol), 124.8mg toluene (0.7mmol), 7.7mg [Cp*RhCl 2 ] 2 (2.5% of the molar dosage of the compound represented by formula II) and 220.0 mg of copper acetate monohydrate (1.1 mmol) were placed in a 25 mL sealed tube containing a magnetic stirrer, and 5 mL of acetone was added. After sealing the sealed tube, put it into an oil bath at 120° C. and stir for 12 hours to perform a cyclization reaction based on carbon-hydrogen bond activation. After the reaction, use petroleum ether-acetone as eluent to carry out column separation to obtain 104.6 mg of a light yellow solid, and the isolated yield of the target product 3-methoxy-5,6-diphenylphenanthroimidazoisoquinoline is 42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com