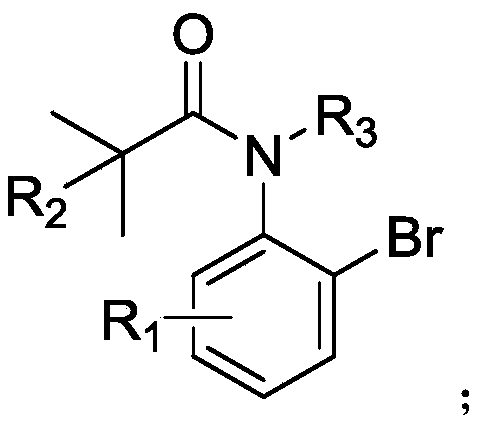
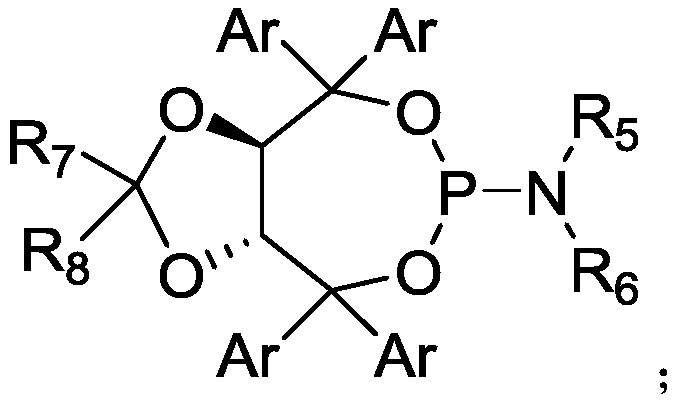
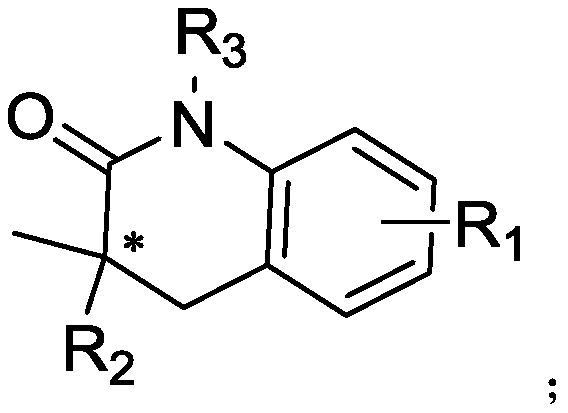
Method for highly enantioselective catalytic synthesis of chiral quinolinone compound
An enantioselective compound technology, applied in the field of high enantioselective catalytic synthesis of chiral quinolinone compounds, can solve the problems hindering the application of drug synthesis research and pharmaceutical industry production, and the asymmetric synthesis method is not popular enough and not very extensive and other problems, to achieve the effect of simplifying the chiral control steps, wide application range and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Add bromoarylamide (0.1 mmol) to a 10 mL Schlenk-type sealed tube equipped with a magnetic stirring bar, (C 3 h 5 )PdCp (10mol%, 2.13mg), L5 (25mol%, 24.7mg), K 2 CO 3 (0.2mmol, 27.6mg), AdCOOH (50mol%, 9.01mg), in N 2 Dry toluene (1 mL) was added under atmosphere. The tube was sealed and reacted at 100°C for 24 hours. After cooling to room temperature, the reaction mixture was filtered through celite and concentrated to obtain a crude product, which was purified by column chromatography, petroleum ether / ethyl acetate=10:1, Rf=0.3, to obtain quinolinone compounds 2a (70% yield, 93% ee).
[0048] white solid. 70% yield. The ee value was analyzed using Daicel Chiralpak ID column, the mobile phase was n-hexane / isopropanol=90 / 10, and the flow rate was 1.0 mL / min. Retention time: 13.2min[(S)-enantiomer], 15.0min[(R)-enantiomer]. 93% ee. [α] 20 D =+73.0(c 0.1,CH 2 Cl 2 ).
[0049] 1 H NMR (400MHz, Chloroform-d) δ7.31(t, J=7.6Hz, 1H), 7.17(d, J=7.6H...
Embodiment 2
[0051]
[0052] Add bromoarylamide (0.1 mmol) to a 10 mL Schlenk-type sealed tube equipped with a magnetic stirring bar, (C 3 h 5 )PdCp (5mol%, 1.06mg), L1 (10mol%, 5.68mg), K 2 CO 3 (0.15mmol, 20.7mg), AdCOOH (30mol%, 5.41mg), in N 2 Dry toluene (1 mL) was added under atmosphere. The tube was sealed and reacted at 100°C for 24 hours. After cooling to room temperature, the reaction mixture was filtered through celite and concentrated to obtain a crude product, which was purified by column chromatography, petroleum ether / ethyl acetate=10:1, Rf=0.3, to obtain quinolinone compounds 2a (0yield).
Embodiment 3
[0054]
[0055] Add bromoarylamide (0.1 mmol) to a 10 mL Schlenk-type sealed tube equipped with a magnetic stirring bar, (C 3 h 5 )PdCp (5mol%, 1.06mg), L2 (10mol%, 6.80mg), K 2 CO 3 (0.15mmol, 20.7mg), AdCOOH (30mol%, 5.41mg), in N 2 Dry toluene (1 mL) was added under atmosphere. The tube was sealed and reacted at 100°C for 24 hours. After cooling to room temperature, the reaction mixture was filtered through celite and concentrated to obtain a crude product, which was purified by column chromatography, petroleum ether / ethyl acetate=10:1, Rf=0.3, to obtain quinolinone compounds 2a (18% yield, 74% ee).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com