Preparation method of anti-tuberculosis candidate drug pa-824
A technology of PA-824, a candidate drug, applied in antibacterial drugs, organic chemistry methods, organic chemistry and other directions, can solve the problems of high price, unfavorable large-scale production, high synthesis cost, and achieves simple operation, easy control, and easy scale. Easy to prepare and purify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
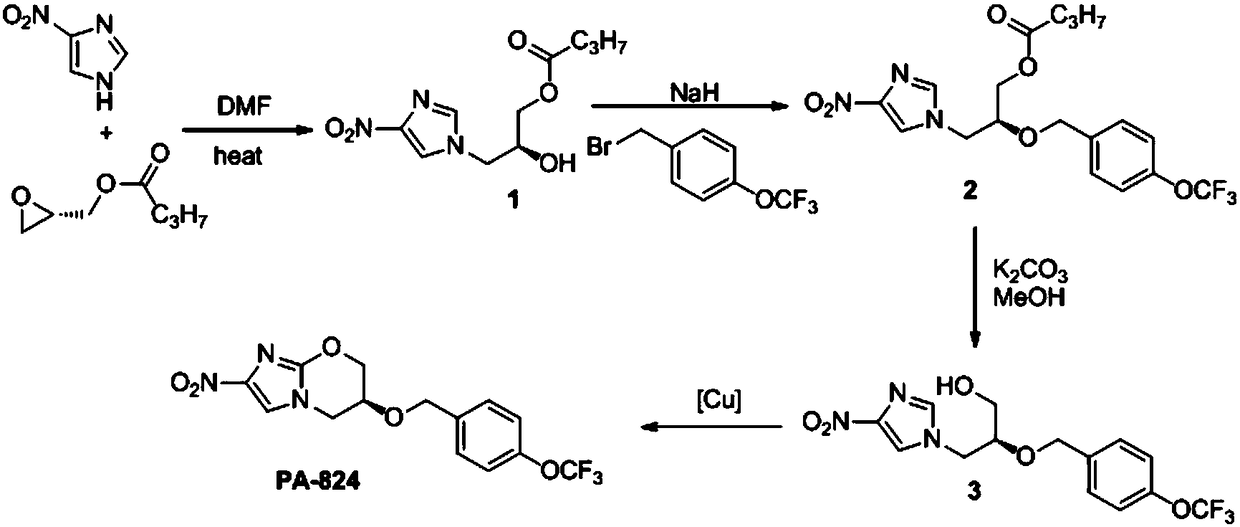
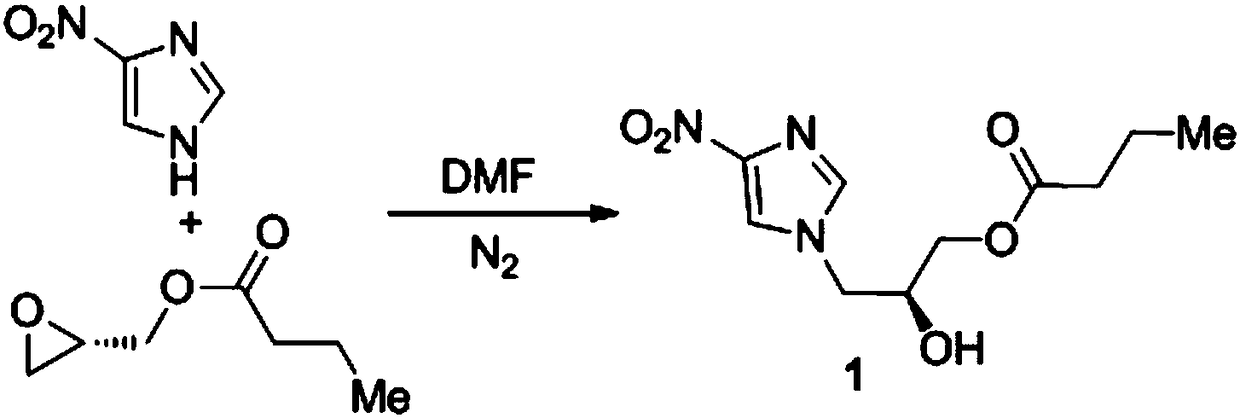
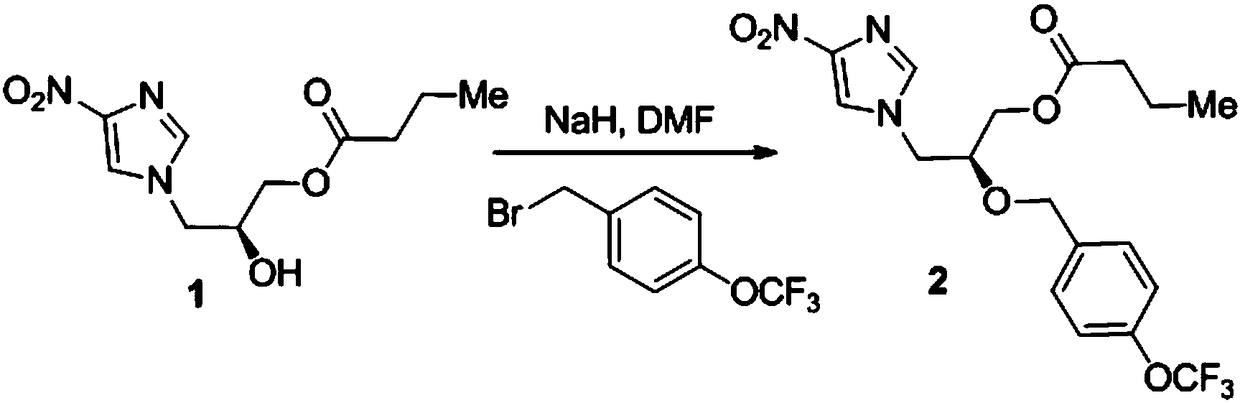
[0019] A synthesis method of nitroimidazole pyran anti-tuberculosis drug candidate PA-824, the steps are as follows:
[0020] (1) In DMF, 4-nitroimidazole and (S)-(+)-glycidylbutyrate with a molar ratio of 1:1.2 undergo nucleophilic ring opening reaction to obtain compound 1:
[0021]
[0022] Under the protection of nitrogen, add 76.5g (0.531mol) (S)-(+)-glycidylbutyrate and 400mL N,N-dimethylformamide (DMF) into a 1000mL three-necked flask, add 50.0g( 0.442mol) solid powder 4-nitroimidazole, mixed uniformly, heated to 70℃ and reacted for 72h, TLC detected that the raw material point disappeared, the reaction solution was poured into 400mL water at 20℃, the aqueous layer was extracted three times with 600mL ethyl acetate, and the organic phases were combined , Washed once with saturated sodium chloride solution, dried with anhydrous magnesium sulfate, filtered, removed the solvent under reduced pressure, and obtained by silica gel (200-300 mesh) column chromatography (ethyl acetat...
Embodiment 2
[0033] A synthesis method of nitroimidazole pyran anti-tuberculosis drug candidate PA-824, the steps are as follows:
[0034] (1) In DMF, 4-nitroimidazole and (S)-(+)-glycidylbutyrate with a molar ratio of 1:1 undergo nucleophilic ring-opening reaction to obtain compound 1:
[0035]
[0036] Under the protection of nitrogen, 63.7g (0.442mol) (S)-(+)-glycidylbutyrate and 400mL N,N-dimethylformamide (DMF) were added to a 1000mL three-necked flask, and 50.0g( 0.442mol) solid powder 4-nitroimidazole, mixed uniformly, heated to 80℃ for 48h, TLC detected the disappearance of the raw material point, the reaction solution was poured into 400mL water at 20℃, the aqueous layer was extracted three times with 600mL ethyl acetate, and the organic phases were combined , Washed once with saturated sodium chloride solution, dried with anhydrous magnesium sulfate, filtered, removed the solvent under reduced pressure, and obtained by silica gel (200-300 mesh) column chromatography (ethyl acetate: pe...
Embodiment 3
[0047] A synthesis method of nitroimidazole pyran anti-tuberculosis drug candidate PA-824, the steps are as follows:
[0048] (1) In DMF, 4-nitroimidazole and (S)-(+)-glycidylbutyrate with a molar ratio of 1:2 undergo nucleophilic ring opening reaction to obtain compound 1:
[0049]
[0050] Under the protection of nitrogen, add 127.4g (0.884mol) (S)-(+)-glycidylbutyrate and 400mL N,N-dimethylformamide (DMF) into a 1000mL three-necked flask, add 50.0g( 0.442mol) solid powder 4-nitroimidazole, mixed well and heated to 60℃ to react for 96h, TLC detected the raw material point disappeared, the reaction solution was poured into 400mL water at 20℃, the aqueous layer was extracted three times with 600mL ethyl acetate, and the organic phases were combined , Washed once with saturated sodium chloride solution, dried with anhydrous magnesium sulfate, filtered, removed the solvent under reduced pressure, and obtained by silica gel (200-300 mesh) column chromatography (ethyl acetate: petroleu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com