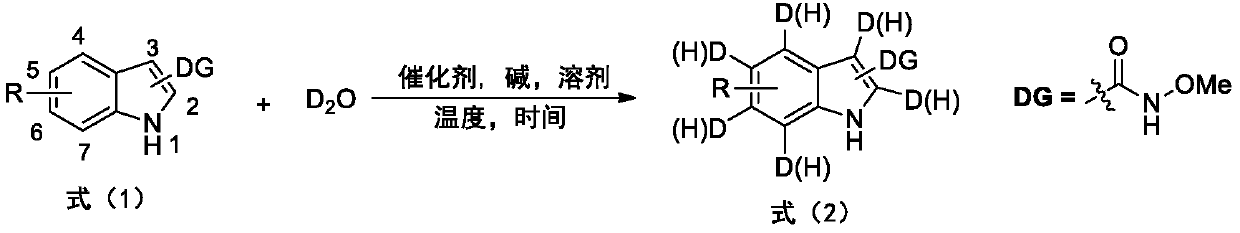
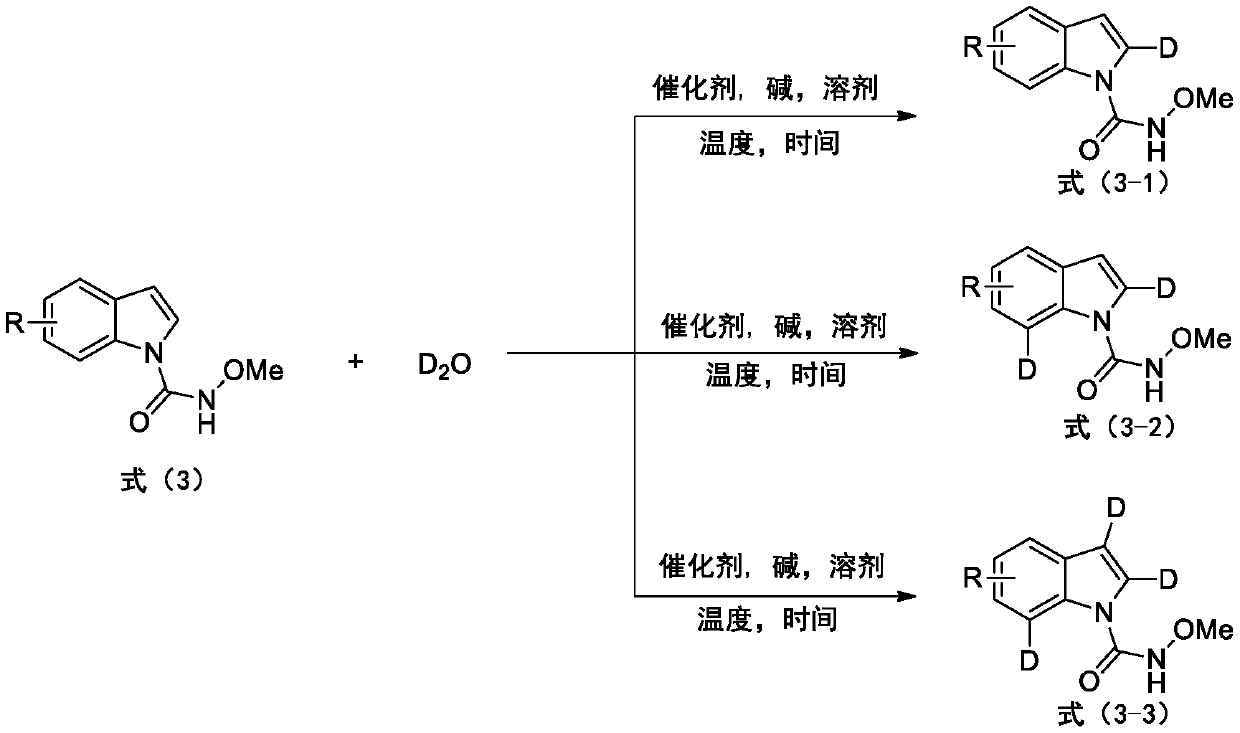
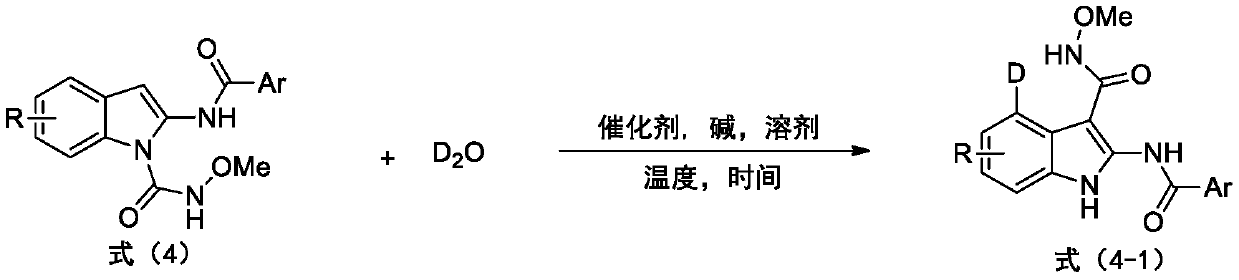
Deuterated synthesis method of indole compound
A synthesis method and compound technology, applied in the field of deuterated synthesis of indole compounds, can solve the problems of poor deuterated position selectivity, severe reaction conditions, expensive deuterium sources, etc., and achieve good substrate applicability, low cost, The effect of high deuteration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 : This embodiment relates to the general synthetic method of the 2-selective deuterated indole compound 3-1a to 3-1p of formula (3-1) by the indole compound shown in formula (3), and its preparation method is According to the following reaction equation.
[0043]
[0044] The substituted indole (0.5mmol) of the N-methoxyamide shown in formula (3), D 2 O(1mL), Cp*Co(Co)I 2 (0.025mmol, 12.0mg), NaOAc (0.5mmol, 41mg) and CH 3 CN (3mL) was added to a 10mL reaction tube containing a stirrer, and the reaction was stirred at 90°C for 12 hours. After cooling to room temperature, the solvent was removed by rotary evaporation, and purified by column chromatography (petroleum ether: ethyl acetate 4:1) to obtain Selective 2-position deuterated indole compounds shown in formula (3-1).
[0045] This example specifically relates to the synthesis method of compound 3-1a.
[0046]
[0047] Using N-methoxyamide indole as raw material, 3-1a was obtained according to the...
Embodiment 2
[0048] Example 2 : The synthetic method of compound 3-1b
[0049]
[0050] According to the same method described in Example 1, using the 3-methyl-substituted N-methoxyamide indole as a raw material, its 2-position deuterated compound 3-1b was obtained. The product detection data is as follows: white solid, yield 80 %; 1 H NMR (500MHz, CDCl 3 ):δ8.31(1H,s),8.13(1H,m),7.51(1H,dt,J=7.5,1.0Hz),7.34(1H,m),7.26(1H,m),3.87(3H, s),2.25(3H,s);HRMS(ESI)m / z calcd for C 11 h 12 DN 2 o 2 [M+H] + 206.1034, found 206.1033.
Embodiment 3
[0051] Example 3 : The synthetic method of compound 3-1c
[0052]
[0053] According to the same method described in Example 1, the 2-position deuterated compound 3-1c was obtained using the 4-position methyl-substituted N-methoxyamide indole as a raw material, and the product detection data were as follows: white solid, yield 77 %; 1 H NMR (500MHz, CDCl 3 ): δ8.59(1H, s), 7.93(1H, d, J=8.0Hz), 7.22(1H, m), 7.04(1H, dd, J=7.5, 0.5Hz), 6.62(1H, d, J=0.5Hz), 3.87(3H,s), 2.51(3H,s); HRMS(ESI) m / z calcd for C 11 h 12 DN 2 o 2 [M+H] + 206.1034, found 206.1030.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com