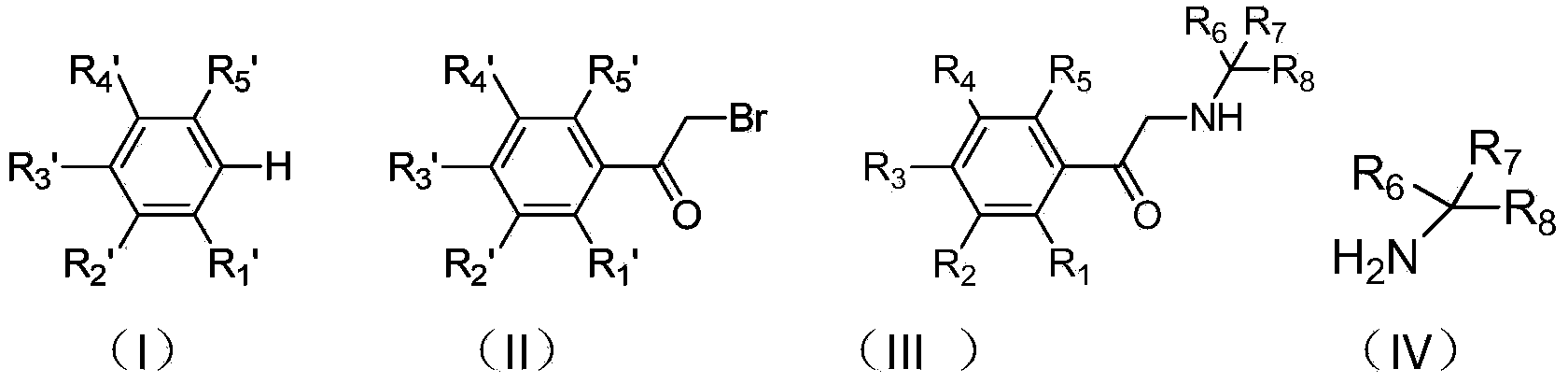
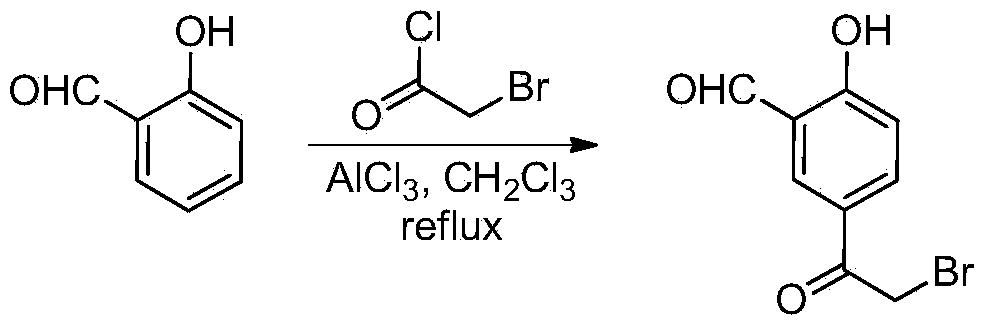
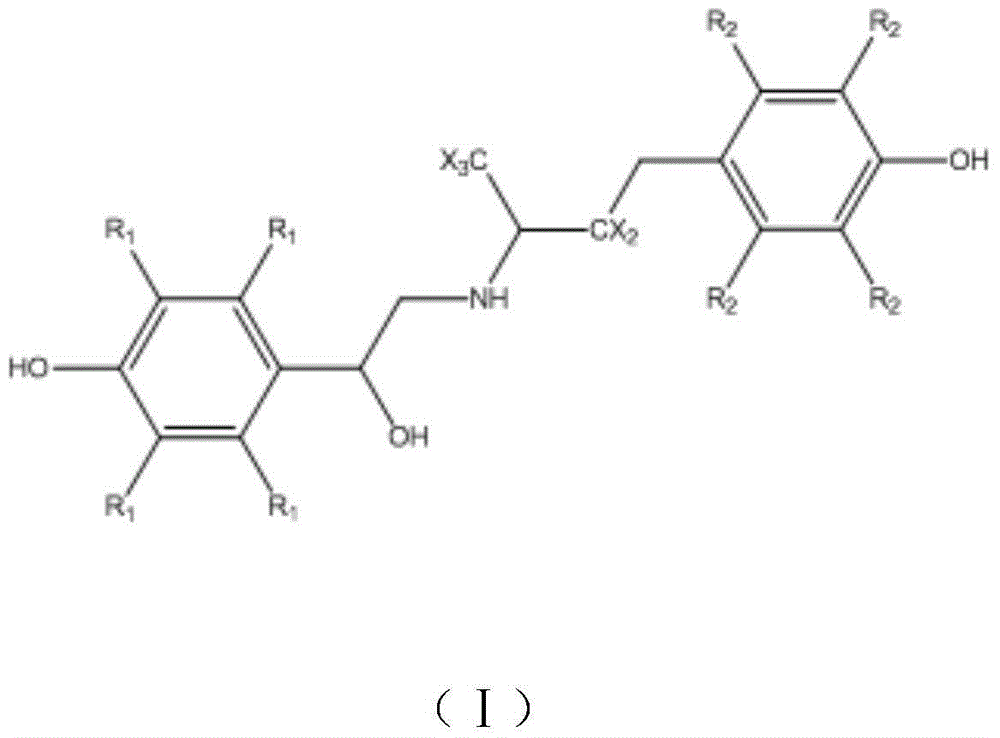
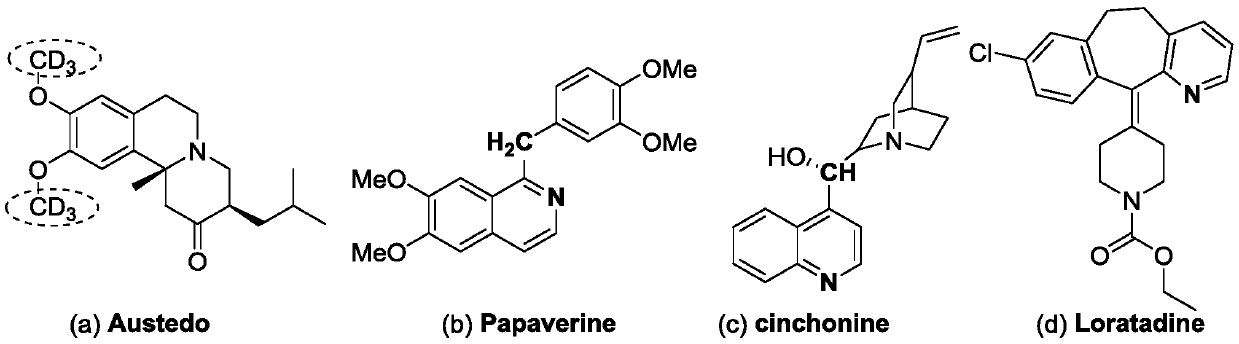
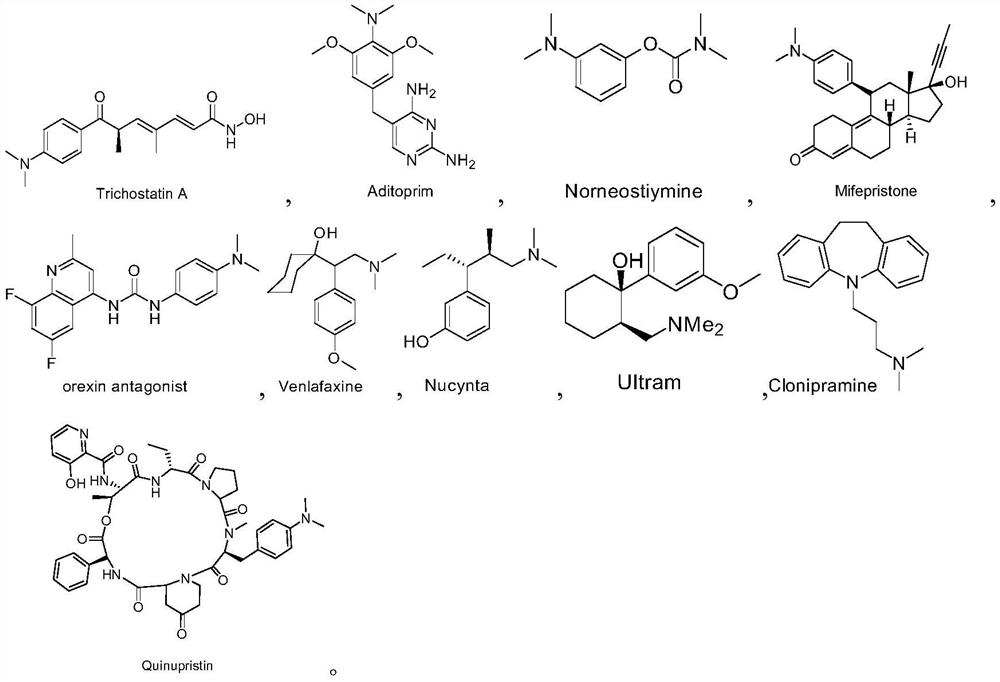
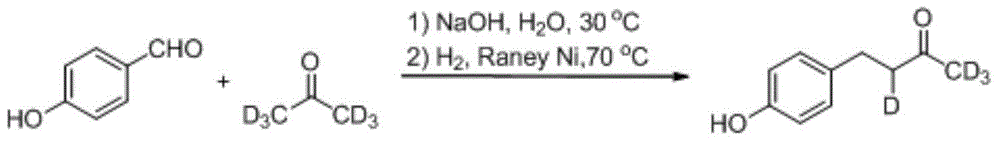Patents
Literature
46results about How to "High rate of deuterium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
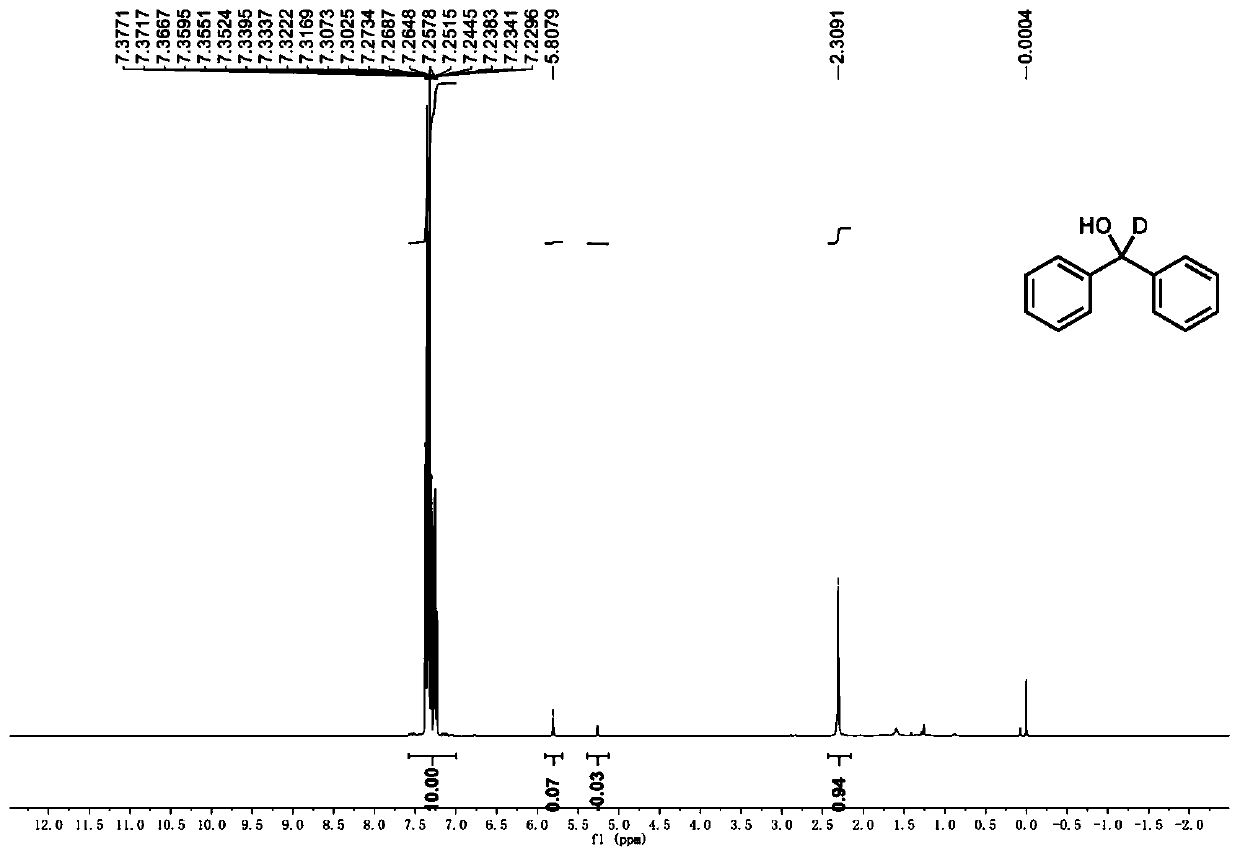
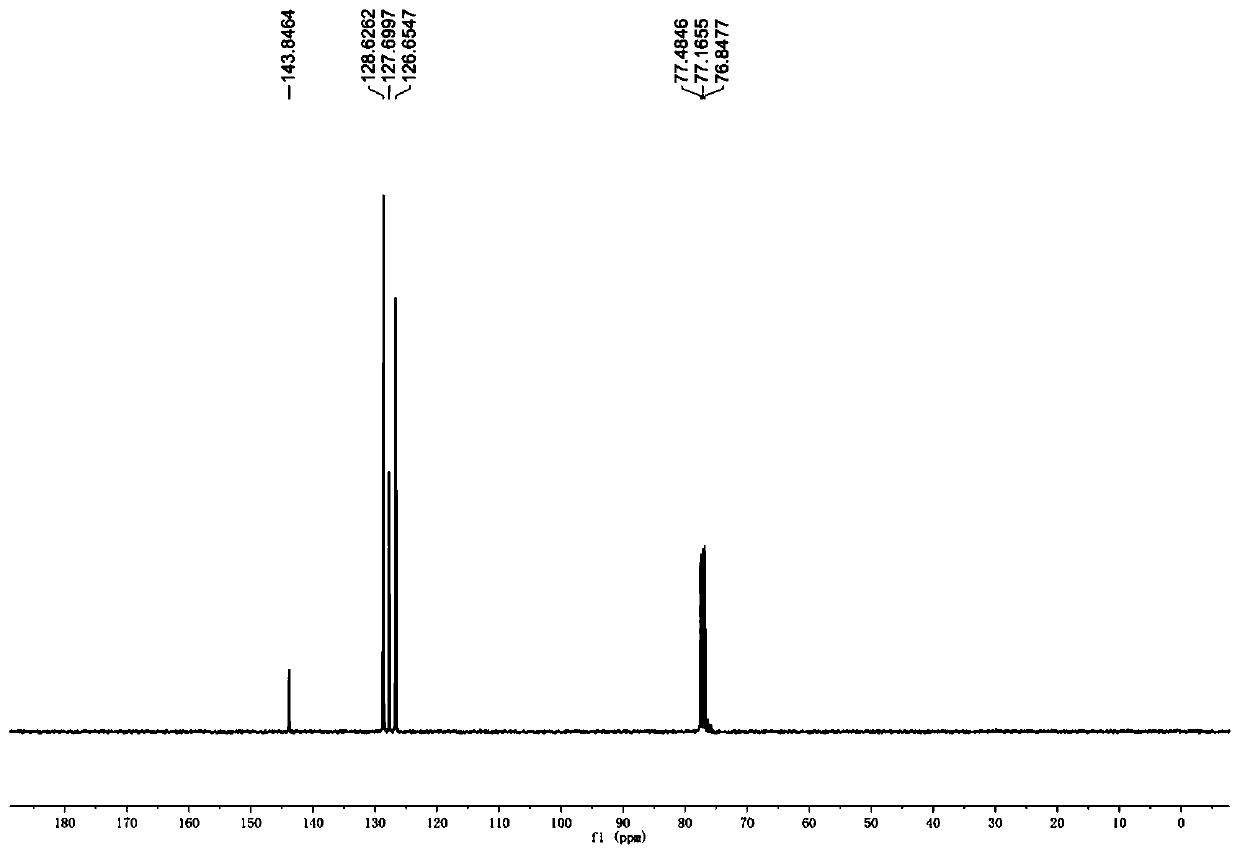
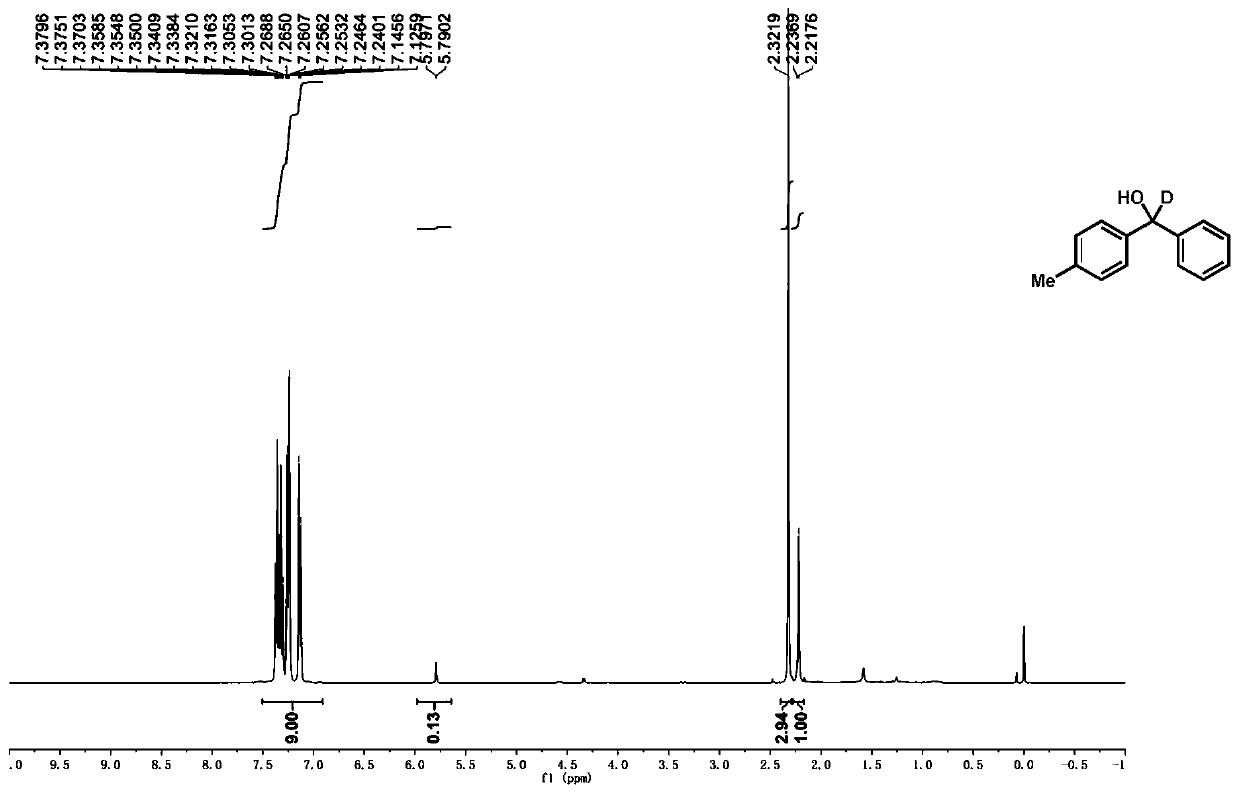
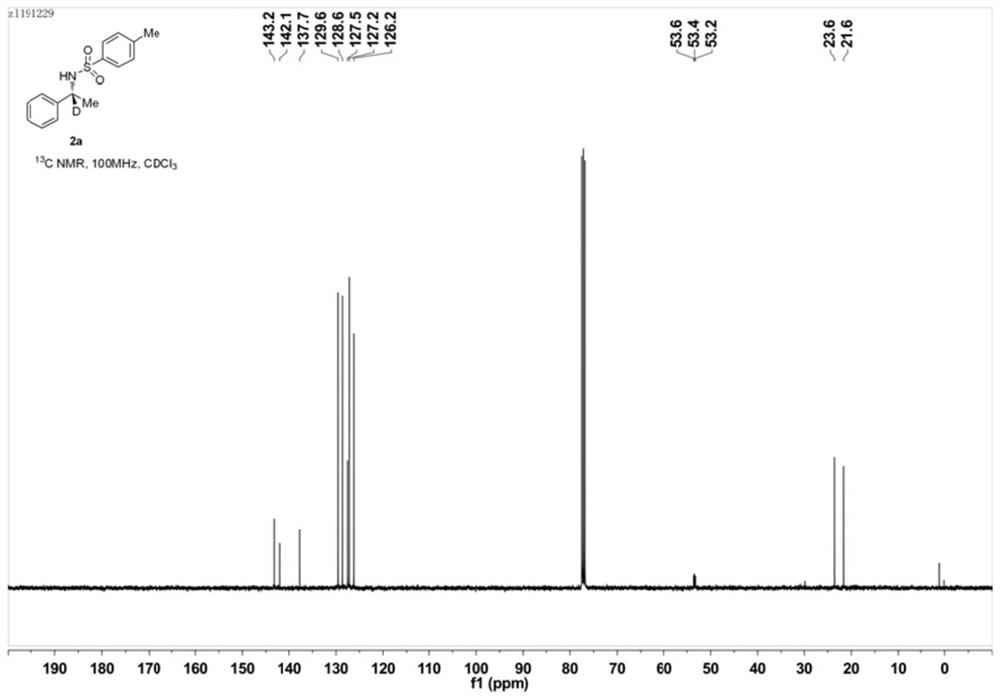
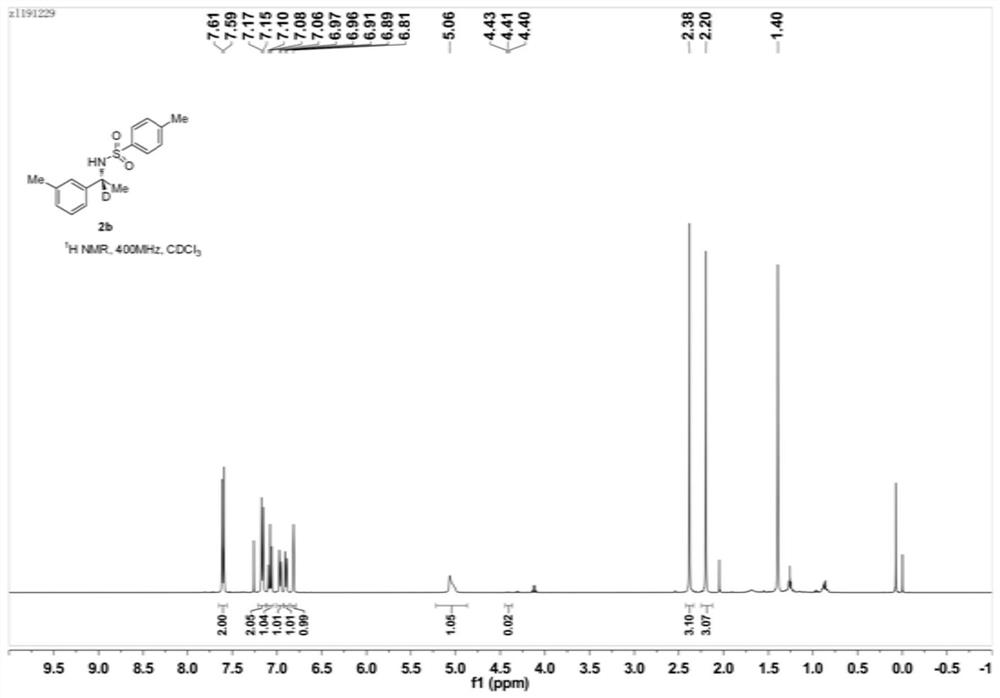
Preparation method of deuterated alcohol or deuterated amine compounds
ActiveCN110128233ALow priceStable in natureIsotope introduction to heterocyclic compoundsOrganic compound preparationElectron donorKetone
Aiming at the defects in the prior art, the invention provides a simple method for producing and preparing deuterated alcohol or deuterated amine compounds. In the method, heavy water is used as a deuterium source, ketone or imine is used as a reaction raw material, and visible light or sunlight is used as an energy source; the reaction can be efficiently carried out at normal temperature and normal pressure. Through selection of a deuterium source, a catalyst and an electron donor, research on reaction conditions and improvement on reagents used in the reaction, the method can overcome the defects of expensive deuterium source, dangerous reaction process, rigorous reaction conditions, low deuteration rate, poor selectivity and the like in the prior art.
Owner:NANJING UNIV OF TECH
Synthetic method of deuterium-labeled phenylethanolamine beta receptor stimulants
InactiveCN104292061AThe synthesis method is simpleHigh rate of deuteriumCarboxylic acid nitrile preparationOrganic compound preparationChemical purityNatural abundance
The invention discloses a method of synthesizing deuterium-labeled phenylethanolamine beta receptor stimulants with high efficiency and a high deuterated ratio. The synthetic method comprises the following steps: performing Friedel-Crafts acylation, performing nucleophilic substitution and performing reduction to prepare pure deuterium-labeled phenylethanolamine beta receptor stimulants. The synthetic method disclosed by the invention is simple and efficient; the synthesized products have chemical purity greater than 99% and labeled point isotope abundance greater than 99%, and can be used for detecting residues of forbidden veterinary drugs such as clenbuterol in the food safety field as well as researching a metabolic mechanism of the forbidden veterinary drugs.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH
Synthesis method for deuterium marked ractopamine
InactiveCN104311436AThe synthesis steps are simpleEasy to control temperatureOrganic compound preparationAmino-hyroxy compound preparationChemical synthesisSynthesis methods
The invention belongs to the technical field of chemical synthesis, and particularly relates to a synthesis method for ractopamine marked by stable isotope deuterium. The method comprises the steps of firstly, carrying out aldol reaction on deuterium marked or non-marked p-hydroxy benzaldehyde and deuterium marked or non-marked acetone so as to generate deuterium marked raspberry ketone, then carrying out reductive amination, carrying out nucleophilic substitution reaction on deuterium marked or non-marked Omega-bromine-hydroxyacetophenone, reducing so as to prepare the deuterium marked ractopamine. The synthesis method has simple and efficient steps, the synthesized deuterium marked ractopamine has the purity of more than 99%, and the mark point isotope abundance is greater than 99%. Furthermore, the synthesis method has high finished product yield and high product yield, and effectively lowers the production cost. The deuterium marked ractopamine prepared by the synthesis method can be used for detection of residue of forbidden veterinary drug in food safety field and research of metabolic mechanism of ractopamine.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH
Method for converting carbon halogen C-R to hydrocarbon/deuterium bond C-H/D of photocatalytic polyhalides
InactiveCN110204425AHigh rate of deuteriumGood choiceOrganic compound preparationCatalystsQuantum rodsHalogen
The invention discloses a method for converting carbon halogen C-R to hydrocarbon / deuterium bond C-H / D of photocatalytic polyhalides. The method includes the following steps: adding a photocatalyst quantum dot / rod into a solvent to obtain a solution A; adding the polyhalides and an electronic sacrificial into the solution A to obtain a solution B; and irradiating the solution B with a light sourcefor catalysis for dehalogenation conversion of the polyhalides. The method is the first to use the nano quantum dots and the nano quantum rods for the dehalogenation conversion reaction of the polyhalides, is mild in reaction conditions and uses visible light as driving energy, products are completely dehalogenated hydrocarbon compounds, and the whole process is green, simple and efficient. The method combines the dehalogenation conversion of the polyhalides and a deuteration labeling process to completely complete the conversion of multiple C-R bonds to C-D bonds to realize the deuteration labeling of polyatoms in one step.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Synthetic method of deuterated hexogen
InactiveCN108101858AHigh purityHigh rate of deuteriumOrganic chemistry methodsHexamethylenetetramineChemistry
The invention relates to a synthetic method of deuterated hexogen, specifically to deuteration technology of hydrogen atoms which are located on non-active carbon-hydrogen bonds inside energetic material molecules. The synthetic method is characterized by reacting a deuterated formaldehyde substance with an ammonia substance to obtain deuterated urotropine which is an intermediate; and further performing nitrification to obtain deuterated hexogen which is the final product. The method is simple and efficient in steps, safe to operate, and is simple in purification. The prepared deuterated hexogen product is high in purity, high in deuteration rate, and suitable for fields of neutron scattering research, energetic material research and nuclear magnetism research.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF +1
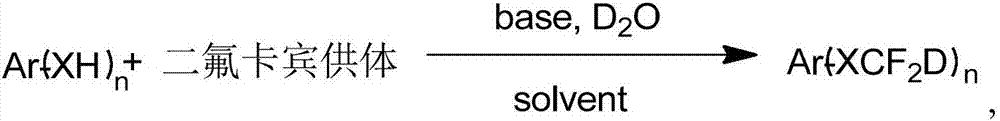
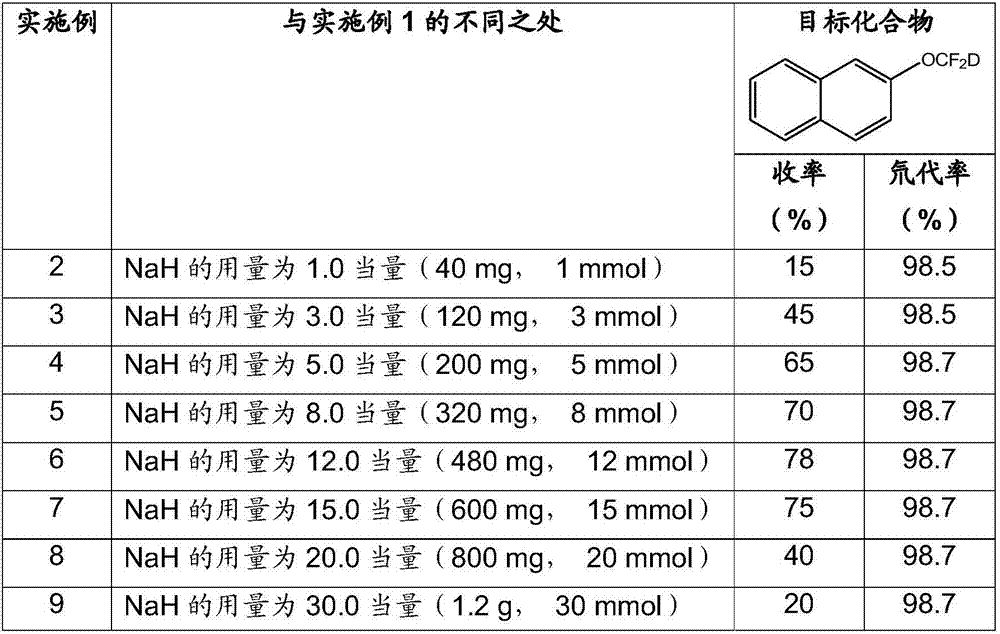
Synthesis method of difluorodeuteromethoxy(thio) function group-containing aromatic compound
InactiveCN107226769AHigh rate of deuteriumHigh yieldIsotope introduction to heterocyclic compoundsCarboxylic acid nitrile preparationThio-Synthesis methods
The invention belongs to the technical field of deuterium-containing compound synthesis and discloses a synthesis method of an aromatic compound containing a difluorodeuteromethoxy functional group or a difluorodeuterothio functional group. In the presence of an alkali, a difluorocarbene donor, a group shown in the description and heavy water undergo a reaction to produce a deuterated product shown in the description, wherein the difluorocarbene donor is BrCF2PO(OR)2 or (chlorodifluoromethyl)trimethylsilane or (bromodifluoromethyl)trimethylsilane and the alkali is selected from Na, K, Li, Mg, Zn, Al, NaH, KH, LiH, LiAlH4, KOD, NaOD, NaOMe, NaOEt, NaOBu, sodium carbonate, potassium carbonate and cesium carbonate. The method has the advantages of use of cheap and easily available raw materials, simple and mild reaction, high yield, high deuteration rate, and production amplification easiness.
Owner:TETRANOV PHARMA CO LTD
N-deuterium methylamine compound and preparation method thereof
PendingCN111302951AHigh rate of deuteriumHigh yieldAmino compound purification/separationIsotope introduction to heterocyclic compoundsPhoto catalysisLight source
The invnetion discloses an N-deuterium methylamine compound and a preparation method thereof. The preparation method comprises the following steps: mixing an amine compound, a deuterium source and a photocatalyst, and carrying out a reaction in an inert gas atmosphere under a light source to obtain the N-deuterium methylamine compound. According to the preparation method, more environment-friendlyand cheap deuterium water and deuterated methanol are used as deuterium sources, the deuterated methanol is used as a deuterium methyl source, and selective N-deuterium methylation reaction of the amine compound at normal temperature and normal pressure is realized by using a photocatalyst under the action of photocatalysis, so that the N-deuterium methylamine compound is prepared. Compared withthe traditional synthesis of alkylamine deuterated products, the method has the advantages of higher selectivity, milder reaction conditions and higher economic applicability.
Owner:SHENZHEN UNIV
Alpha, alpha-dideuterated alcohol compound and preparation method thereof
PendingCN112679312AMild reaction conditionsEasy to operateOrganic compound preparationHydroxy group formation/introductionAlcoholOrganosolv
The invention relates to a method for synthesizing an alpha, alpha-dideuterated alcohol compound shown in a general formula (2), which is characterized in that an amide compound shown in a general formula (1) reacts with a bivalent lanthanide transition metal compound, a deuterium donor reagent and Lewis base in an organic solvent I to generate the alpha, alpha-dideuterated alcohol compound shown in the general formula (2) . compared with the traditional method, the method has the advantages of strong selectivity, high yield, high deuteration rate, few toxic and side products, low cost, mild reaction conditions, simplicity in operation, environmental friendliness and the like.
Owner:北京奇点势能科技有限公司
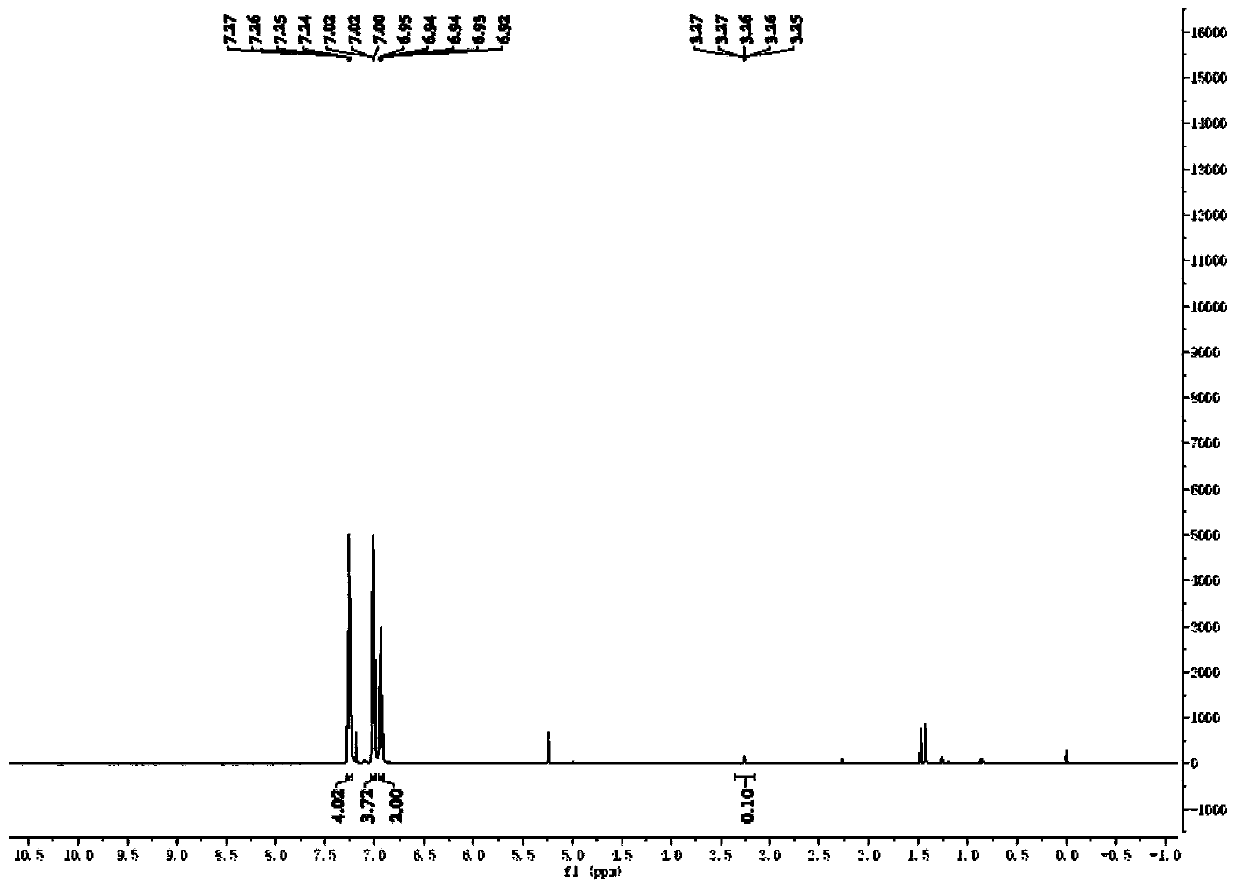
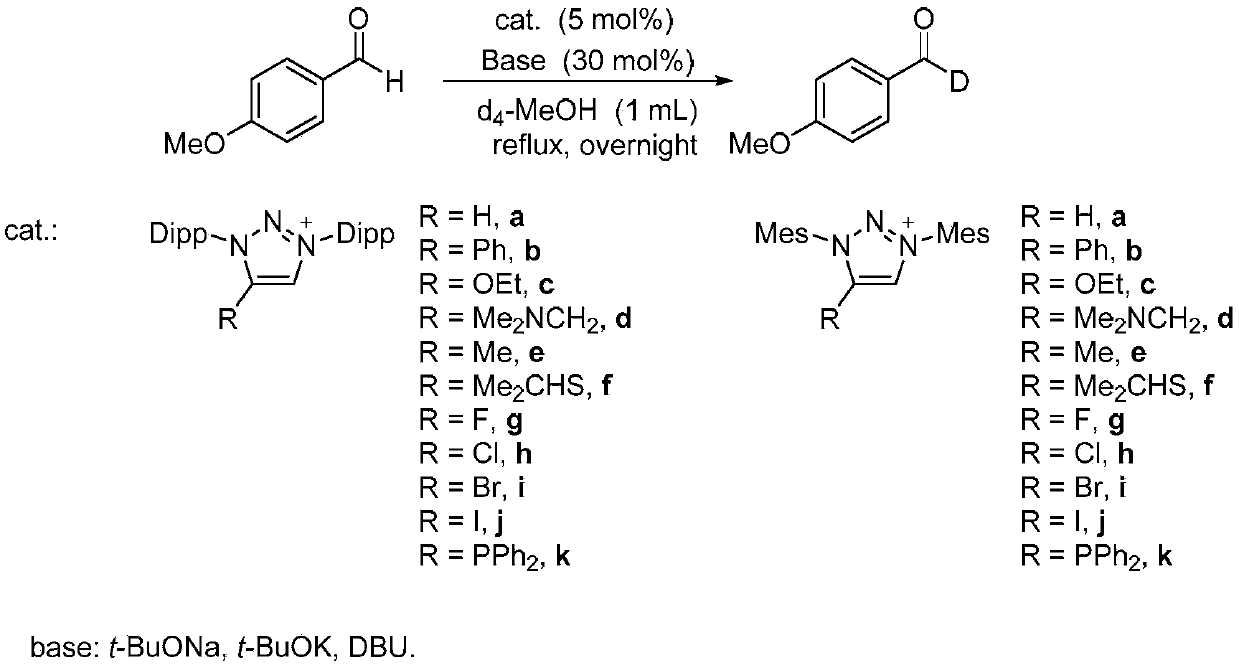
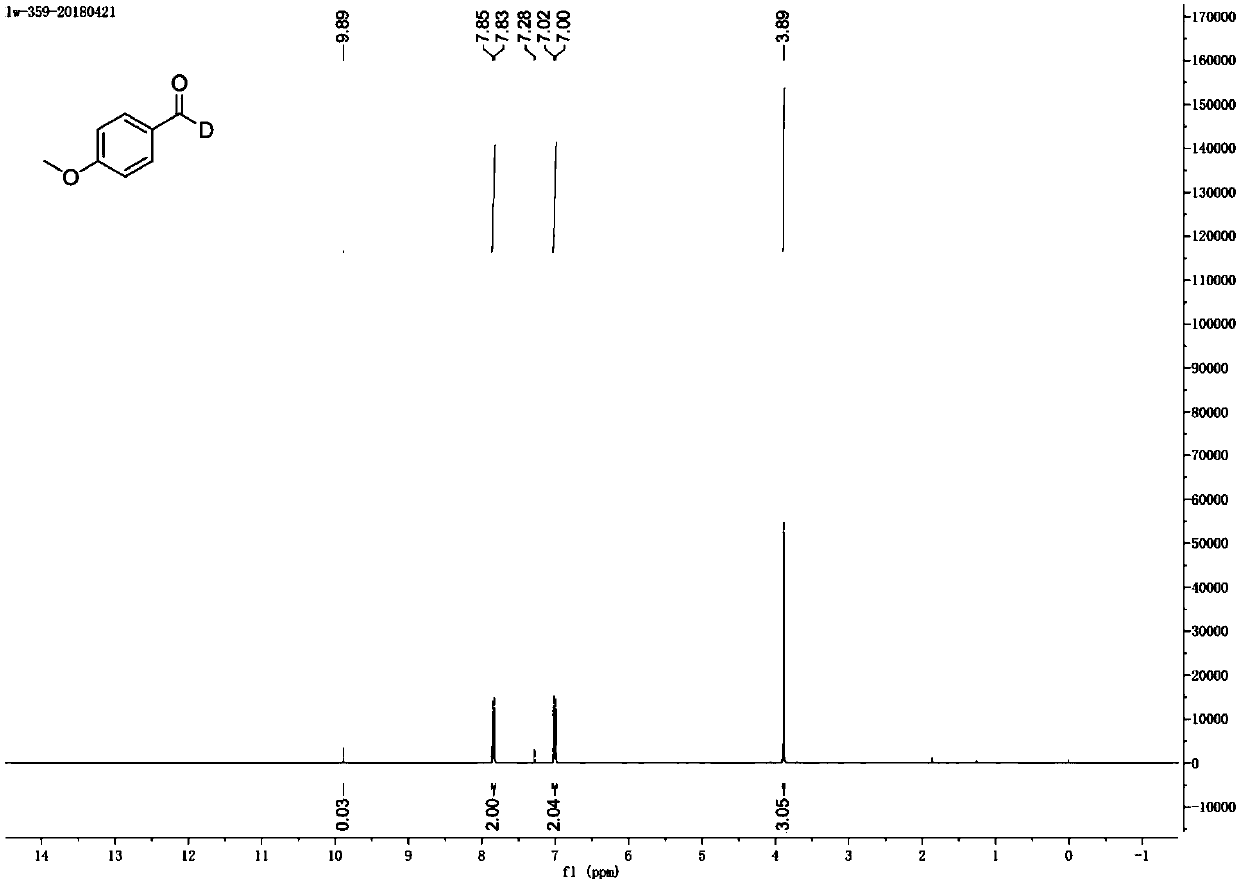
Method for preparing deuterated aldehyde through triazole carbene catalysis
ActiveCN111039767AHigh rate of deuteriumImprove applicabilityOrganic compound preparationOrganic chemistry methodsArylPtru catalyst
The invention provides a novel method for preparing deuterated aldehyde, which comprises the following step: by using deuterated methanol as a deuteration reagent, carrying out a hydrogen-deuterium exchange reaction on an aldehyde in the presence of a catalyst and alkali to obtain the deuterated aldehyde. According to the method, the deuterated methanol is used as the deuteration reagent, a triazole carbene salt is used as a catalyst precursor, deuteration of the aldehyde is effectively realized under the action of an alkali, and the deuteration rate of the aldehyde is as high as 98%. Moreover, the applicability of the reaction substrate is wide, deuteration of aryl aldehydes can be realized, deuteration of alkyl and alkenyl aldehydes can be realized, and medium to good deuteration rates can be obtained in the reactions. According to the metal-catalysis-free hydrogen-deuterium exchange reaction, hydrogen-deuterium exchange of the reaction is achieved through a manner of activating aldehyde with carbene, reaction steps are reduced, the atom economy is improved, and the applicability of a substrate is wide. Carbene is used as a catalyst, expensive precious metal does not need to be used as a catalyst, the reaction cost is reduced, and the reaction has very high economical efficiency and applicability.
Owner:RENMIN UNIVERSITY OF CHINA
Safe, environment-friendly and cheap method and device for producing deuterated aromatic ring compound
ActiveCN111099955AHigh rate of deuteriumLow costDistillation purification/separationHydrocarbonsPtru catalystSimple aromatic ring
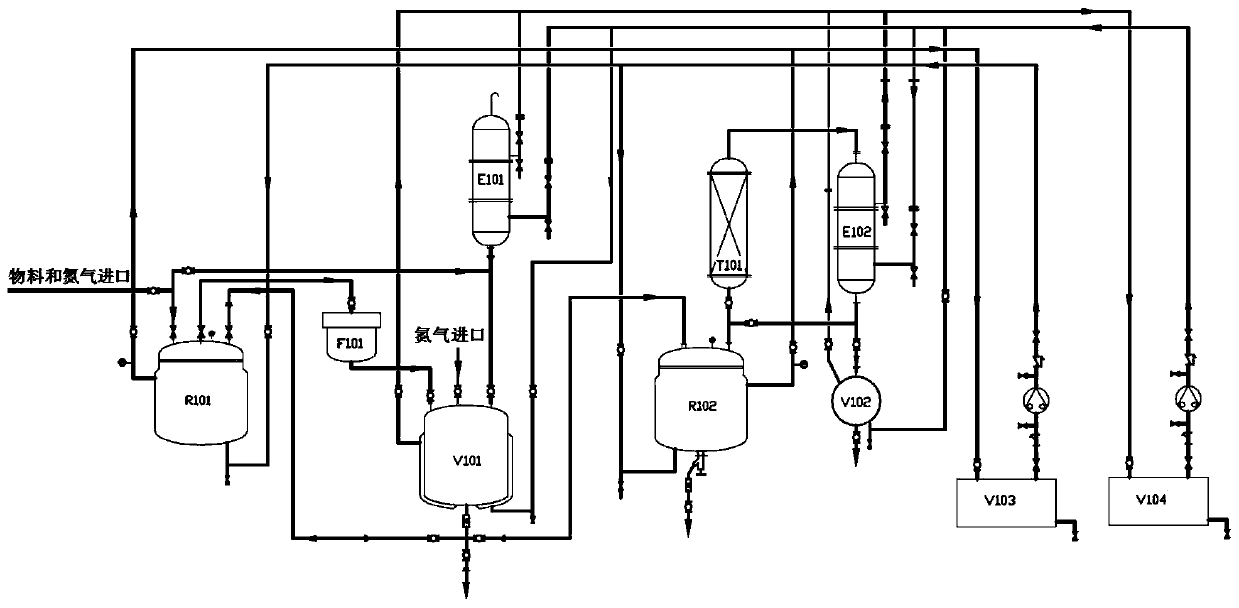
The invention provides a safe, environment-friendly and cheap method and device for producing a deuterated aromatic ring compound. The device is mainly composed of a reaction system, a post-treatmentsystem, a rectification system and a heat exchange system. By adopting the device, the yield of the deuterated aromatic ring compound can be increased to 95%, and the cost is reduced to 74.74% of theoriginal cost. For a product with stronger volatility under lower boiling point, the effect is more obvious when the device is used. Therefore, after the device is used, the volatilization loss of theproduct can be avoided, the cost is reduced, meanwhile, personnel harm and environmental pollution caused by volatilized toxic deuterated aromatic ring compounds are basically avoided, and the problems of safety and environmental protection cost are solved. According to the method, the product yield is increased, the heavy hydrogen utilization rate is increased and the catalyst is recycled and reused through a set of designed equipment and experimental scheme, finally, the aromatic ring compound with the high deuteration rate can be produced, and in industrial production, along with increaseof production batches, the cost can be further saved, and the yield is increased.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
High-selectivity deuteration method of 2-methyl azacyclo compound
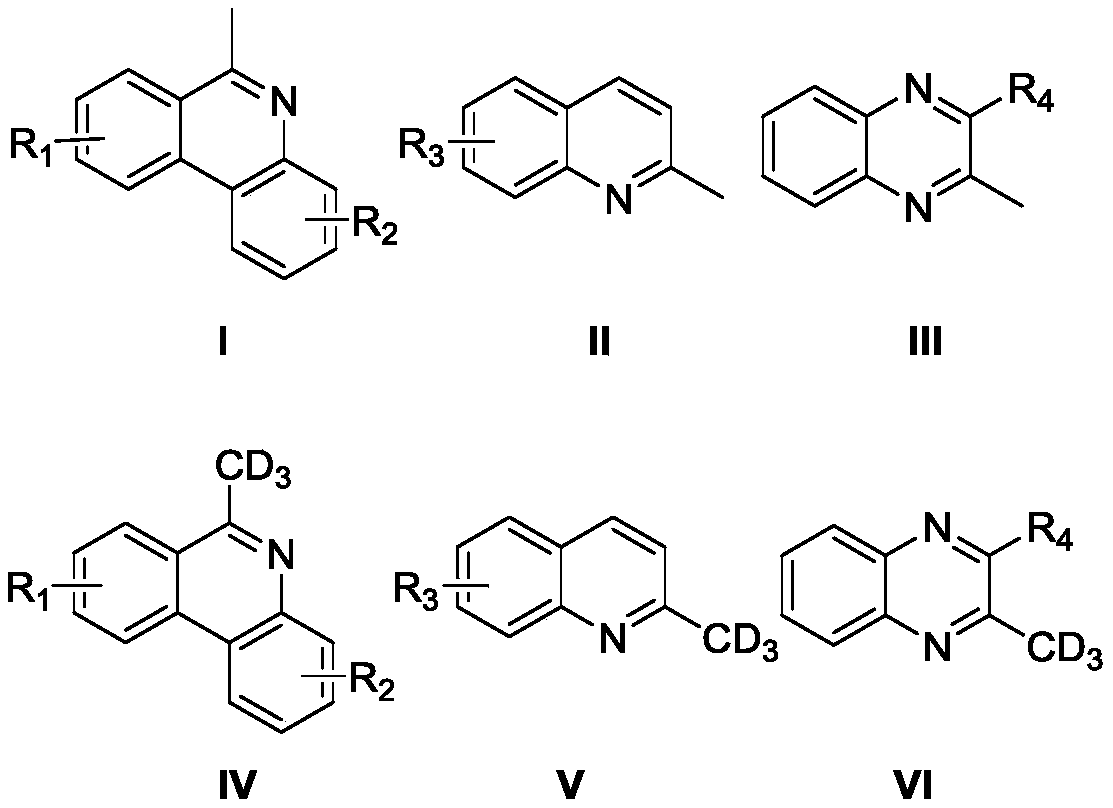
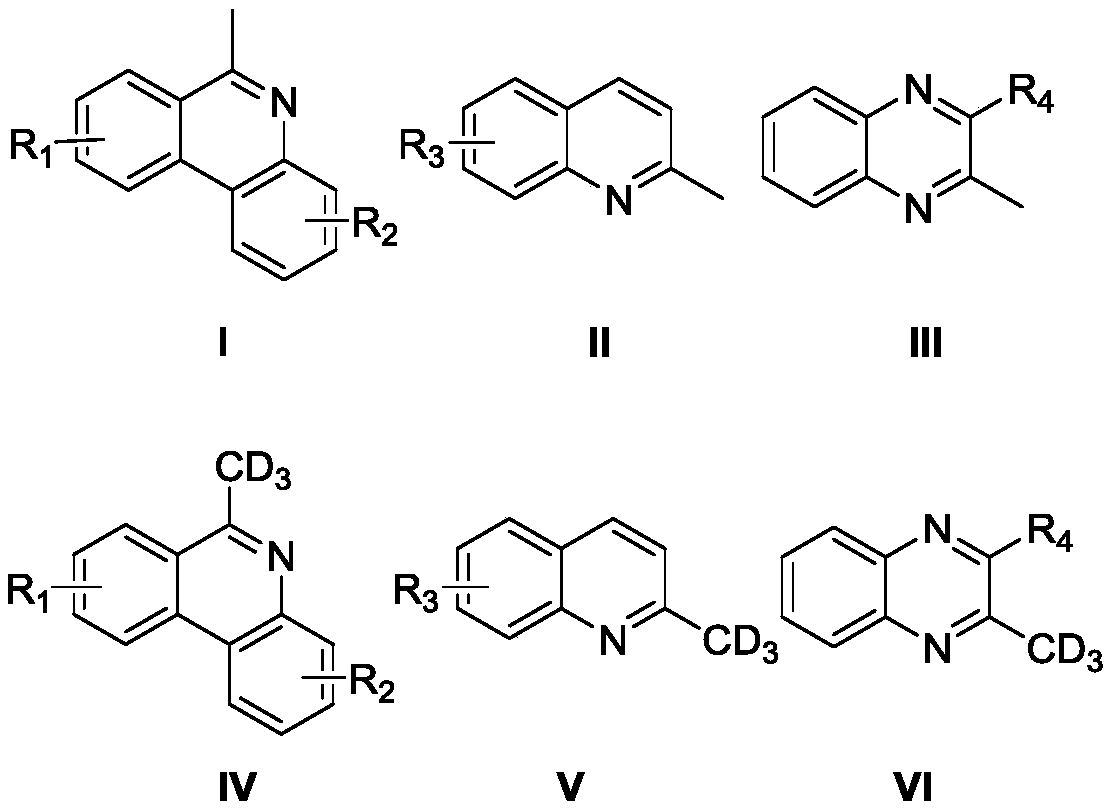
ActiveCN110563649AReduce consumptionUniversalIsotope introduction to heterocyclic compoundsShielding gasHigh selectivity
The invention discloses a high-selectivity deuteration method of a 2-methyl azacyclo compound. The method is carried out according to the following steps: adding a 2-methyl azacyclo compound shown asa formula I, a formula II or a formula III, an oxidant and an additive into a dry Schlenk reaction tube; and in the presence of a protective gas, adding deuterium water and an organic solvent into thereaction tube, performing a stirring reaction at 50-100 DEG C for 2-12 hours to obtain a reaction solution, and performing post-treatment on the reaction solution to respectively obtain a deuteratednitrogen-containing heterocycle shown in a formula IV, a formula V or a formula VI. The method provided by the invention is based on a free radical process, is efficient, can synthesize methyl-d3 substituted nitrogen-containing heterocyclic compounds which are difficult to prepare by conventional methods, and is high in deuteration rate of reaction; the method is carried out under a neutral condition and has low requirements on equipment; a catalytic amount of an oxidant is used, and additives are cheap and easily available; reaction conditions are mild, and energy consumption is reduced; theyield is high, the substrate universality is strong, the operation is simple and convenient, and the like.
Owner:ZHEJIANG UNIV OF TECH
Alpha, alpha-dideuterated benzyl alcohol compound, deuterated medicine and reduction deuteration method of benzoate compound
PendingCN113354513AHigh rate of deuteriumGood location selectivityIsotope introduction to heterocyclic compoundsCarbamic acid derivatives preparationBenzoic acidOrganic solvent
The invention relates to an alpha, alpha-dideuterated benzyl alcohol compound and a reduction deuteration method of a benzoate compound for preparing the alpha, alpha-dideuterated benzyl alcohol compound. The alpha, alpha-dideuterated benzyl alcohol compound is characterized in that a benzoate compound as shown in a general formula (1) reacts with a divalent lanthanide transition metal compound, a deuterium donor reagent and Lewis base in an organic solvent I to generate the alpha, alpha-dideuterated benzyl alcohol compound as shown in a general formula (2). With the reduction deuteration method of the invention adopted, the defects of low deuteration rate, poor regioselectivity, poor chemical selectivity and need of an expensive ruthenium catalyst or an expensive and flammable metal deuteride in a preparation method of the alpha, alpha-dideuterated benzyl alcohol compound in the prior art can be eliminated. The method has the advantages of high product deuteration rate, good deuteration site regioselectivity, good chemical selectivity, low reagent price, simple operation, mild conditions and wide substrate application range.
Owner:北京奇点势能科技有限公司
Dofetilide-d3 drug and preparation method thereof
PendingCN111393338AHigh rate of deuteriumHigh yieldOrganic compound preparationOrganic chemistry methodsPtru catalystDocetaxel
The invention discloses a dofetilide-d3 drug and a preparation method thereof. The preparation method comprises the following steps: reacting p-chlorophenol with 1, 2-dibromoethane in ethanol at the temperature of 100 DEG C in the presence of sodium hydroxide serving as alkali to obtain a compound III; reacting the compound III with 4-chlorophenylethylamine in a second solvent at 80 DEG C in the presence of alkali to obtain a compound I; reacting the compound I with deuterium sources under the catalysis of a light source and a photocatalyst to obtain a compound II, wherein the deuterium sources comprise deuterium water and d4-deuterated methanol; and reacting the compound II with methanesulfonamide under the catalysis of a palladium catalyst to obtain the docetaxel-d3 drug. According to the preparation method, more environment-friendly and cheap deuterium water and d4-deuterated methanol are used as deuterium sources, the d4-deuterated methanol is used as a deuterium methyl source, andselective N-deuterium methylation reaction on a prodrug amine compound is realized at normal temperature and normal pressure under the action of photocatalysis of a photocatalyst, so that the docetaxel-d3 drug is prepared.
Owner:SHENZHEN UNIV
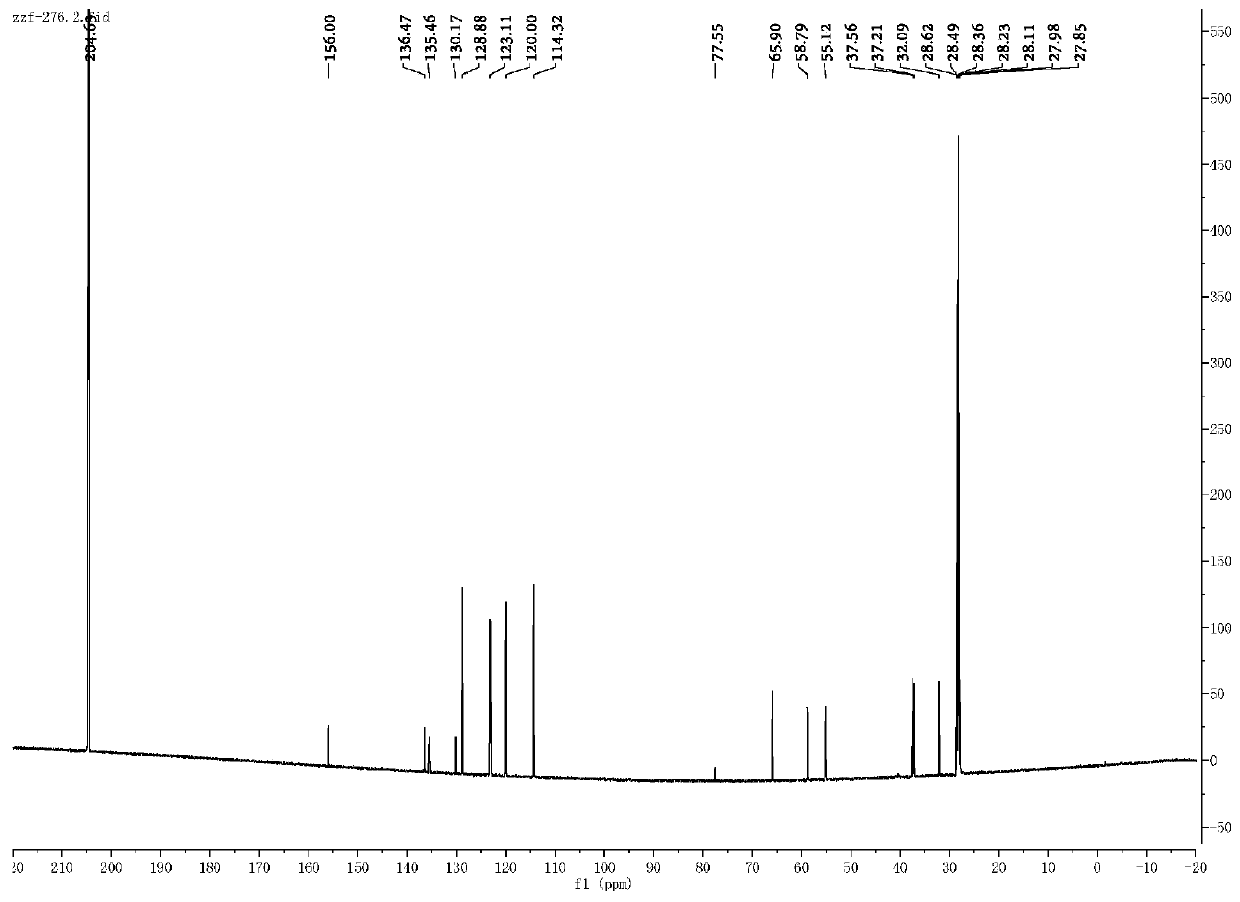
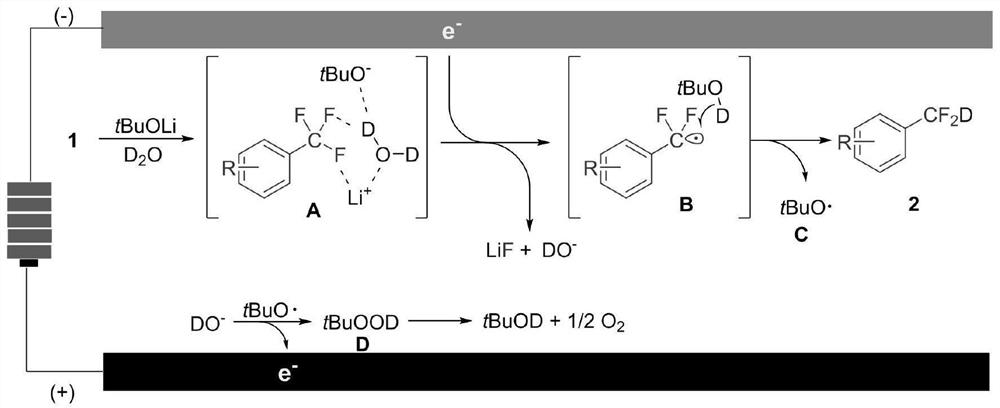
Synthesis method of aryl deuterated difluoromethyl compound
ActiveCN114032568AHigh yieldSimple preparation stepsElectrolysis componentsElectrolytic organic productionArylMeth-
The invention relates to the technical field of organic synthesis, and provides a synthesis method of an aryl deuterated difluoromethyl compound. The aryl deuterated difluoromethyl compound is synthesized by taking an aryl trifluoromethyl compound as a raw material and heavy water as a deuterium source, activating a C-F bond in the aryl trifluoromethyl compound through an electrolytic reaction and introducing a deuterium atom. The deuterium water is used as the only deuterium source, other deuterated reagents are not needed, any transition metal is not used, and the method is suitable for synthesizing drugs sensitive to heavy metal residues. An electrochemical method is used for preparation, an additional reducing agent is not needed, the whole preparation process is simple in step and easy to operate, the product yield is high, and the deuteration rate is high. The method provided by the invention is wide in functional group application range, can synthesize various aryl deuterated difluoromethyl compounds with different functional groups, is suitable for later modification of drugs, and is beneficial to grafting to modification of existing drugs.
Owner:NANXIN PHARM TECH RES INST CO LTD +1
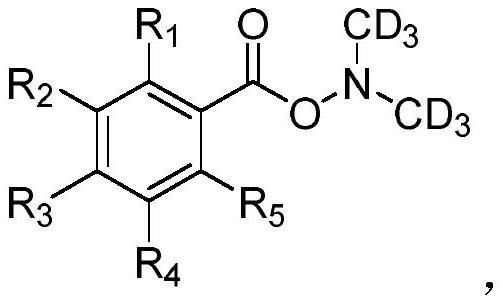
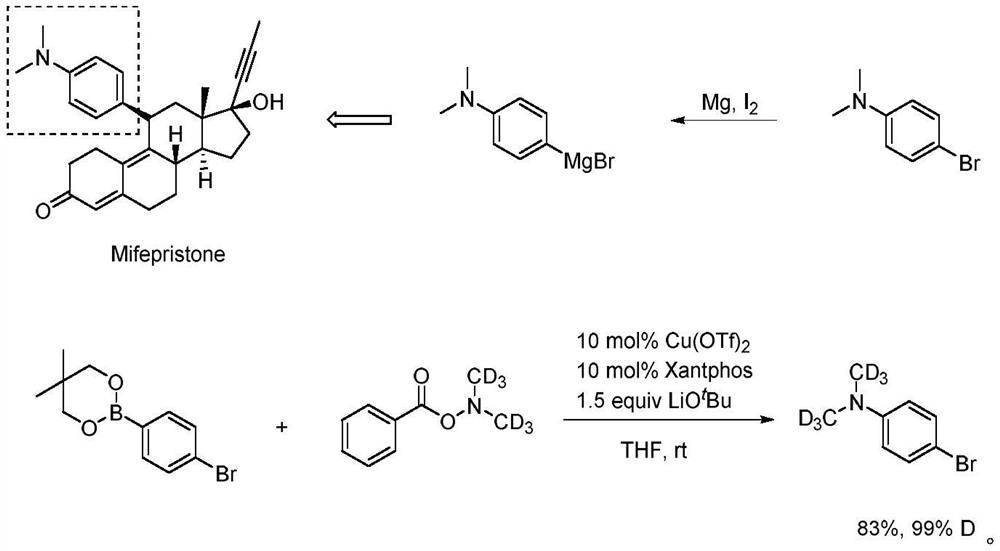
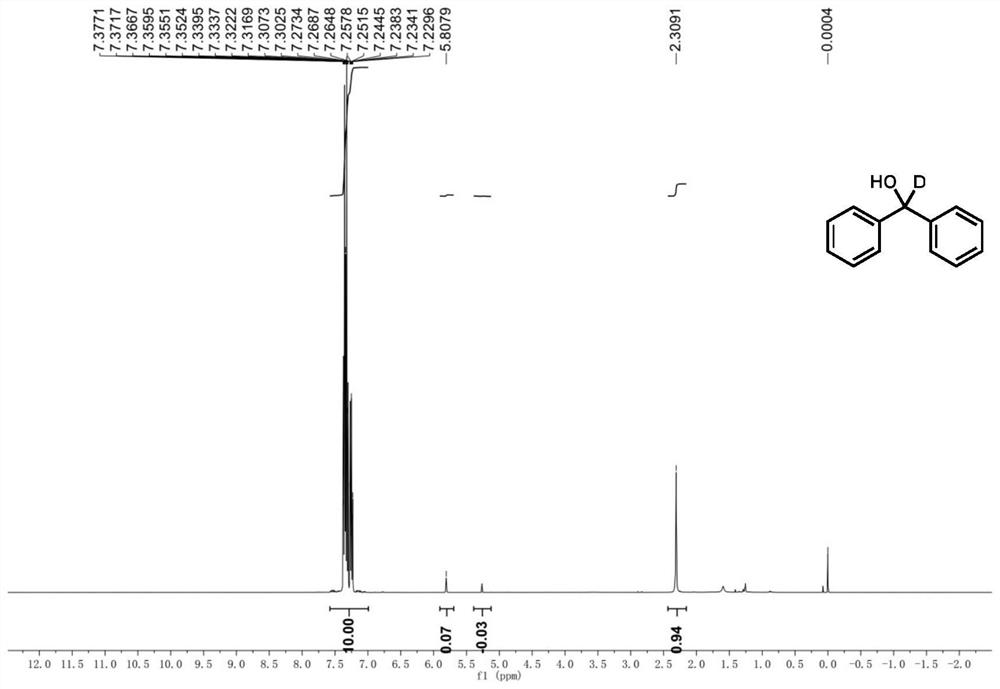
A kind of deuterated dimethylhydroxylamine benzoate compound and its preparation method and application
ActiveCN109265374BPromote generationMild conditionsCarbamic acid derivatives preparationOrganic compound preparationBenzoic acidFormic Acid Esters
The invention provides a deuterated dimethylhydroxylamine benzoate compound and a preparation method and application thereof. The compound has the following structural formula as shown in the description, wherein R1, R2, R3, R4 and R5 represent hydrogen or , methoxyl or , benzyloxy or, methyl or, phenyl or, fluorine or, chlorine or, bromine or, trifluoromethyl, or oxytrifluoromethyloxy or, nitrylor, cyano or sulfonyl separately and independently; the method comprises the preparation steps that in the presence of inorganic salt, deuterated dimethylamine salt and benzoyl peroxide or a derivative of the benzoyl peroxide are protected from light react in an organic solvent, at 15-35 DEG C without light, reaction is carried out for 15-20 h, and then quenching reaction is carried out; the inorganic salt is alkali metal salt. The compound can be used as a deuterated dimethylamine agent for participating in synthsis of deuterated dimethylamine aryl compounds. A The synthesis strategy has thecharacteristics that the conditions are mild, the yield is high, the reaction rate is high, a the deuterated ratio is high, and the like.
Owner:NANJING UNIV
A kind of preparation method of deuterated alcohol compound
ActiveCN110128233BLow priceStable in natureIsotope introduction to heterocyclic compoundsOrganic compound preparationPtru catalystElectron donor
Aiming at the defects and deficiencies in the prior art, the purpose of the present invention is to provide a simple method for the production and preparation of deuterated alcohols or deuterated amines. In the method, heavy water is used as a deuterium source, ketone or imine is used as a reaction raw material, visible light or sunlight is used as an energy source, and the reaction can be efficiently carried out at normal temperature and pressure. Through the selection of deuterium sources, catalysts, electron donors, research on reaction conditions and improvement of reagents used in the reaction, defects such as expensive deuterium sources, dangerous reaction process, harsh reaction conditions, low deuterium substitution rate and poor selectivity in the prior art can be improved. .
Owner:NANJING TECH UNIV
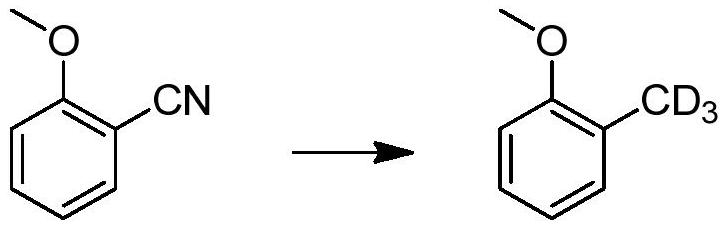
A method for catalytically converting cyano groups into deuterated methyl groups, prepared aromatic deuterated methyl compounds and applications thereof
ActiveCN107353176BImprove pharmacodynamicsReduce metabolic toxicityIsotope introduction to heterocyclic compoundsOrganic compound preparationPtru catalystMethyl palmoxirate
The invention provides a method for catalytic conversion of a cyano group into deuterated methyl, a prepared aromatic deuterated methyl compound and an application of the compound. The method comprises the steps as follows: an aromatic cyano compound reacts to produce the aromatic deuterated methyl compound under the action of a metal catalyst with deuterium gas serving as a deuterium source. The cyano group is directly catalyzed into deuterated methyl with the deuterium gas serving as the deuterium source, the operation is simple, the raw material is cheap and easy to obtain, the reaction yield is high, the product deuteration rate is high, and the method can be applied to mass production. The prepared aromatic deuterated methyl compound can be used as a deuterated medicine or can be used for preparation of a deuterated medicine or deuterated medicine composition, and the pharmacokinetics, pharmacodynamics or metabolism toxicity of the medicine can be reduced while the medicine molecular activity is kept unchanged basically.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Synthetic method of alpha-deuterated carbonyl compound
PendingCN114773174AEasy to operateOperational securityCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystRegioselectivity
The invention belongs to the technical field of preparation of deuterated compounds, and particularly relates to a synthesis method of alpha-deuterated carbonyl compounds. Inorganic base barium oxide BaO or barium hydroxide Ba (OH) 2 is used as a catalyst, a deuterium supply reagent is used as a deuterium source, and deuteration of the alpha position of the carbonyl compound is efficiently completed. According to the method disclosed by the invention, the H / D conversion reaction can be carried out at the alpha position of carbonyl at high selectivity without influencing other positions, the applicable substrates are wide, and the target product can be obtained at high yield and high deuteration rate in both cyclic ketone and chain ketone reactions. For the reaction of nitrile compounds and sulfoxide compounds, the target product can be obtained with high yield and medium deuteration rate, and the method has the characteristics of simplicity, high efficiency and good regioselectivity.
Owner:CHANGZHOU UNIV
A kind of method that takes halomethyl compound as raw material to prepare deuterated aldehyde
ActiveCN108383697BHigh yieldSimple reaction conditionsIsotope introduction to heterocyclic compoundsCarboxylic acid nitrile preparationMeth-Nitrobenzene
The invention provides a method for preparing deuterated aldehydes by using halomethyl compounds as raw materials. Described method comprises the steps: in the solvent that pyridine compound exists and D 2 Add halomethyl compounds to O mixed liquid, and react at 0°C~100°C; then add nitrosobenzene compounds to continue the reaction, and finally the reaction liquid is acidified to obtain the target product. The raw materials used in the method of the present invention are all cheap and easy to obtain, no expensive reagents or heavy metal raw materials are required, and the reaction conditions are simple, without harsh conditions such as high temperature, that is, the preparation method of the present invention is low in cost, simple in operation, and high in deuterium substitution rate. Up to 97%, the target product is easy to purify and the yield is high, up to 95%. Moreover, the substrate of the method of the present invention has wide applicability, and various deuterated aldehyde compounds can be prepared.
Owner:SUN YAT SEN UNIV
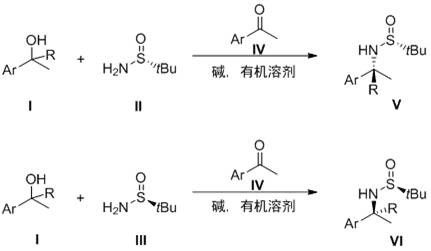
A kind of preparation method of nickel-catalyzed α-deuterated chiral sulfonamide compound
ActiveCN111233716BReduce dosageReduce usageIsotope introduction to heterocyclic compoundsSulfonic acid amide preparationPtru catalystAcyl group
The invention discloses a method for preparing a nickel-catalyzed α-deuterated chiral sulfonamide compound. Under an inert atmosphere, a chiral complex is used as a catalyst, and a deuterated alcohol is used as a deuterium source and a solvent. Under ℃, N-sulfonyl imine undergoes asymmetric transfer deuteration reaction to obtain α-deuterated chiral sulfonamide compound; the chemical structural formula of N-sulfonyl imine is: α-deuterated chiral sulfonamide compound The chemical structural formula is: where, R 1 , R 2 , R 3 from C 1 -C 6 Alkyl, aryl, substituted aryl, heterocyclic aryl, R 1 and R 2 Different; the chiral complex is generated in situ by divalent nickel and chiral phosphine ligands. The invention can not only overcome the shortcomings of the existing transition metal catalyzed deuteration reaction, but also has the advantages of environmental friendliness, simple operation, high reaction efficiency, wide substrate applicability and the like.
Owner:SHANDONG NORMAL UNIV
Catalytic production process of deuterated benzene
InactiveCN112778072AHigh rate of deuteriumOrganic chemistry methodsHydrocarbonsActivated carbonPlatinum
The invention discloses a catalytic production process of deuterated benzene, which comprises the following steps of: mixing benzene and heavy water according to a volume ratio of 1: 2, and adding platinum on activated carbon; and carrying out sealed heating and stirring reaction at 100-130 DEG C for 8-15 hours, separating, and distilling to obtain the target product deuterated benzene, wherein the platinum carbon is added at a speed of 15-35% / min based on the total weight. A manual powder valve is specially designed for the test, and the adding speed of platinum on activated carbon is controlled through the manual powder valve, so that the influence of the adding speed of platinum on activated carbon into the mixed solution of benzene and heavy water on the deuteration rate of the final target product is researched; and experimental research finds that the deuteration rate of the target product is related to the adding speed of platinum on activated carbon, and when the adding speed of platinum on activated carbon is 15-35% / min, the deuteration rate of the target product is the highest and exceeds 90%.
Owner:徐州亚兴医疗科技有限公司
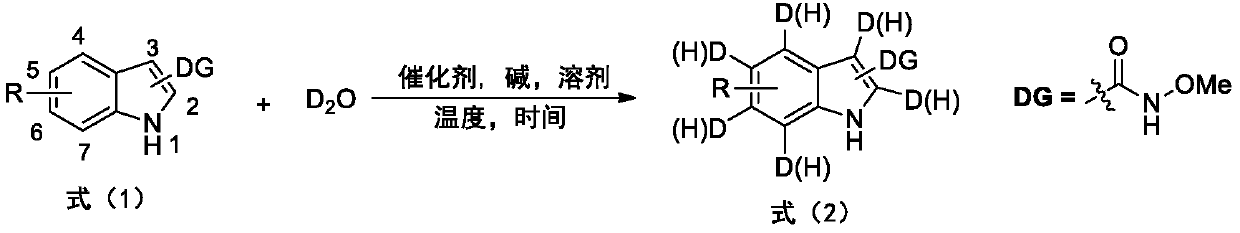
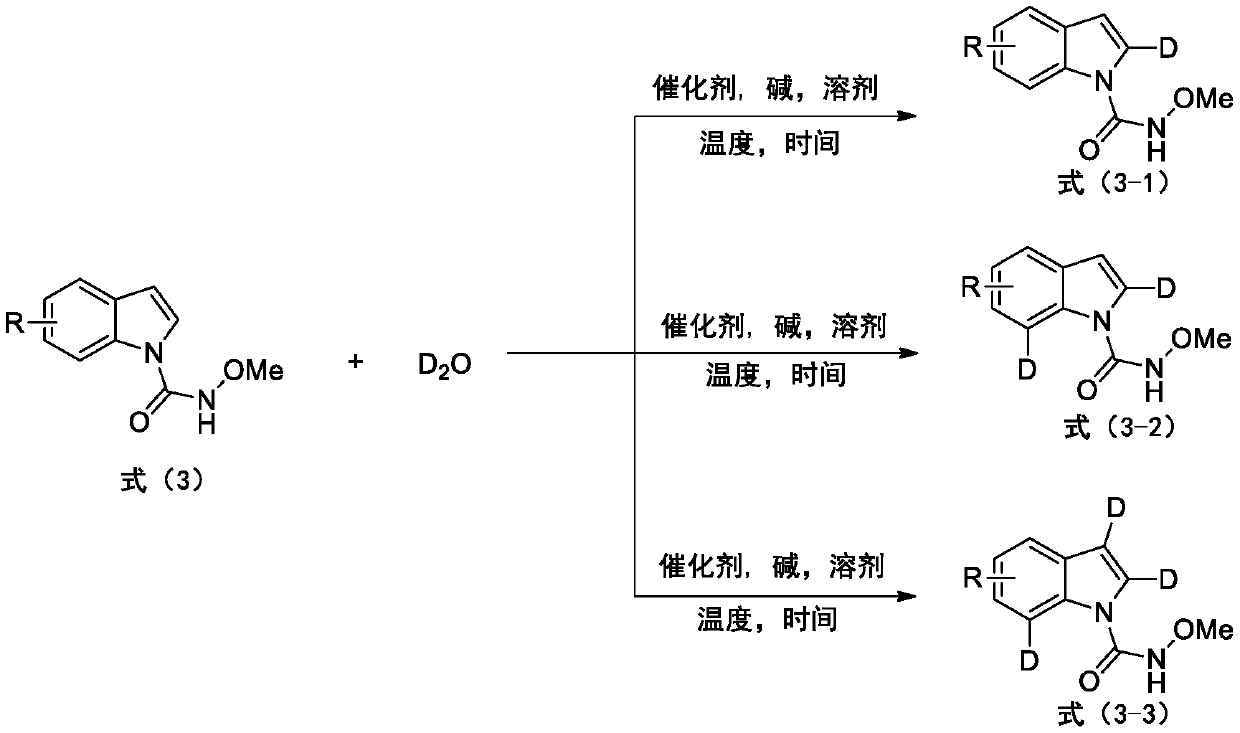
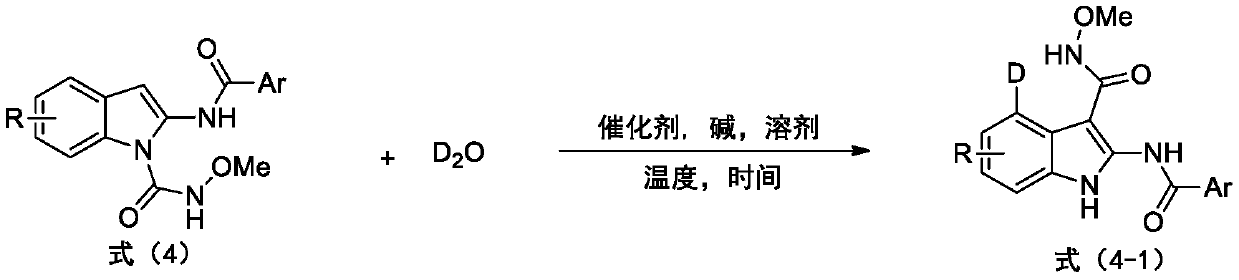
Deuterated synthesis method of indole compound
ActiveCN111533676AMild reaction conditionsEasy to getIsotope introduction to heterocyclic compoundsPtru catalystReaction temperature
The invention provides a deuterated synthesis method of an indole compound, which comprises the following steps: carrying out hydrogen-deuterium exchange on the indole compound through a carbon-hydrogen bond activation reaction guided by a guide group DG at a proper reaction temperature for proper time under the mixed reaction of heavy water, a catalyst, alkali and a solvent to generate a deuterated indole product. The method provided by the invention is mild in reaction condition, simple in process, low in cost, good in deuteration position selectivity and high in yield and deuteration rate,moreover, relatively cheap heavy water is used as a deuterium source, so that the use of an expensive deuterium source in the preparation process of the deuterated compound is effectively avoided, andthe preparation cost of the deuterated compound can be further reduced.
Owner:ZHEJIANG UNIV
A kind of synthetic method of toluene-d8
ActiveCN111440041BHigh rate of deuteriumReduce dosageHydrocarbon purification/separationHydrocarbon from halogen organic compoundsPtru catalystHydrogen exchange
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Synthetic method of alpha-monodeuterated alcohol compound and deuterated drug
InactiveCN112939732AHigh rate of deuteriumGood location selectivityIsotope introduction to heterocyclic compoundsCarboxylic acid nitrile preparationDeuterated drugAlcohol
The invention provides an alpha-monodeuterated alcohol compound and a reduction deuteration method of an aldehyde ketone compound for preparing the alpha-monodeuterated alcohol compound. The aldoketone compound shown in a general formula (1) reacts with a divalent lanthanide transition metal compound and a deuterium donor reagent in an organic solvent I to generate the alpha-monodeuterated alcohol compound shown in a general formula (2). The invention establishes a reduction deuteration method of an aldehyde ketone compound based on a single electron transfer reduction deuteration reaction, and the method is used for preparing the alpha-monodeuterated alcohol compound shown in a general formula (2) as well as deuterated derivatives of aldehyde ketone drugs, hormones and natural products of the alpha-deuterated alcohol compounds. The method has the advantages of high product deuteration rate, good regioselectivity, good chemical selectivity, low reagent price, simple operation, mild conditions and wide substrate application range. Compared with an existing H / D exchange method, the deuterium donor reagent is small in dosage, the cost can be remarkably reduced, and the utilization rate of deuterium atoms is increased.
Owner:CHINA AGRI UNIV
Preparation method of o-position deuterated benzoic acid compound
ActiveCN108191754AHigh selectivityWide range of applicationsIsotope introduction to heterocyclic compoundsPreparation from carboxylic acid amidesBenzoic acidFiltration
The invention discloses a preparation method of an o-position deuterated benzoic acid compound. The preparation method sequentially comprises the following steps: carrying out an oil bath reaction on8-aminoquinoline substituted benzoic acid amide used as a raw material under a sealing state at a temperature of 120-160 DEG C for 24-72 hours in the presence of a deuterated reagent and a palladium catalyst, cooling the obtained reaction liquid to room temperature, carrying out extraction, washing the obtained organic layer, and carrying out drying and concentration; carrying out silica gel column chromatography on the obtained concentrate; carrying out an oil bath reaction on the obtained deuterated intermediate in a sulfuric acid water solution at 120+ / -20 DEG C until a TLC detection reaction is completed; cooling the obtained reaction solution to room temperature, carrying out extraction, washing the obtained ether layer, and carrying out drying, suction filtration and concentration toobtain the o-position deuterated benzoic acid compound. The o-position deuterated benzoic acid compound prepared by adopting the method provided by the invention has the technical advantages of highyield, good selectivity and good economical efficiency.
Owner:ZHEJIANG UNIV
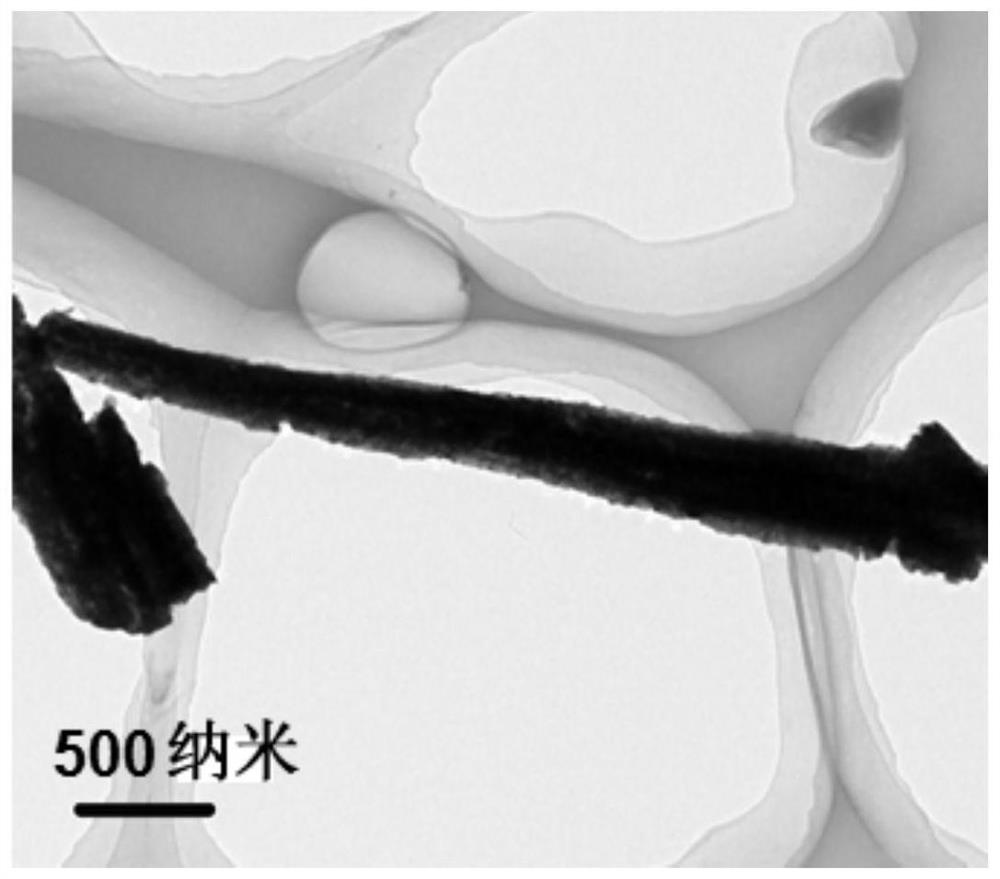
In situ synthesis of copper nanowire array material and its preparation method and application
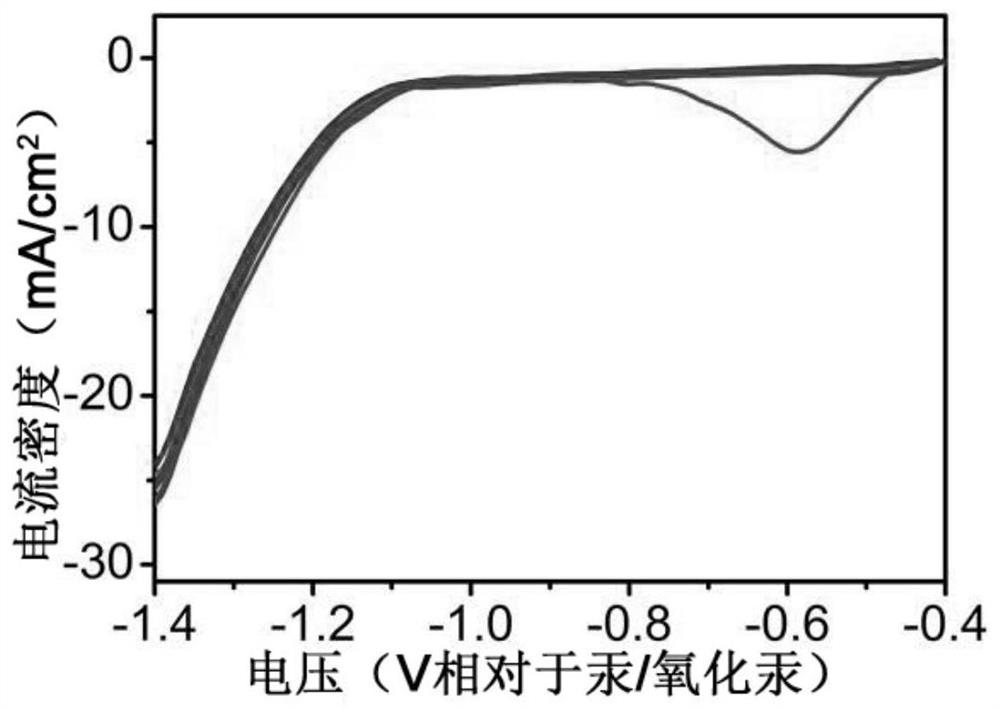
ActiveCN113322490BImprove efficiencyHigh rate of deuteriumMaterial nanotechnologyPhotography auxillary processesElectrochemical responseAir atmosphere
The invention provides an in-situ synthesized copper nanowire array material and its preparation method and application. The copper oxide nanowire array is placed in a separate two-chamber reaction electrolytic cell, and the copper oxide nanowire array is used as a working electrode in an air atmosphere. Mercury / mercury oxide is the reference electrode, the platinum sheet is the counter electrode, and the electrolytic solution is 0.5M K 2 CO 3 A mixed solution of acetonitrile and deuterated water, wherein the volume of acetonitrile accounts for 30-70% of the total volume of the mixed solution, the volume of deuterated water accounts for 30-70% of the total volume of the mixed solution, and the scanning speed is 8-12mV / s, the scanning voltage is in the range of -0.4--1.4V, and the cyclic voltammetry test is carried out until the reduction peak of copper oxide disappears, that is, the in-situ synthesized copper nanowire array material is obtained. The present invention synthesizes the copper nanowire array (Cu NWAs) material by the method of in-situ transformation, takes the copper nanowire array (Cu NWAs) material as the cathode of the electrochemical reaction, utilizes a kind of simple and convenient electrochemical reduction method to deuterate water The efficient conversion of C-X / C-D is realized for the deuterium source.
Owner:TIANJIN UNIV
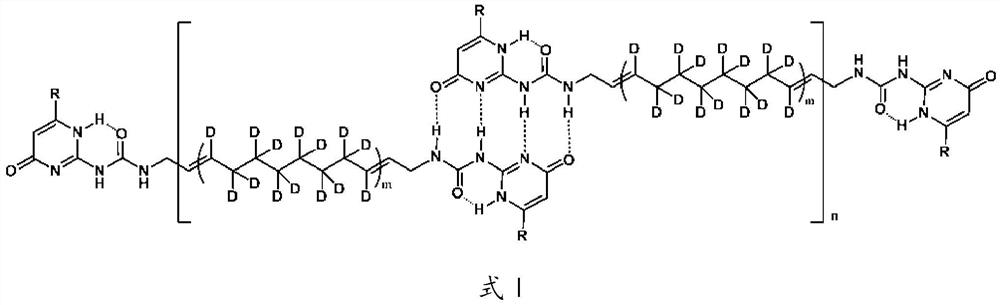
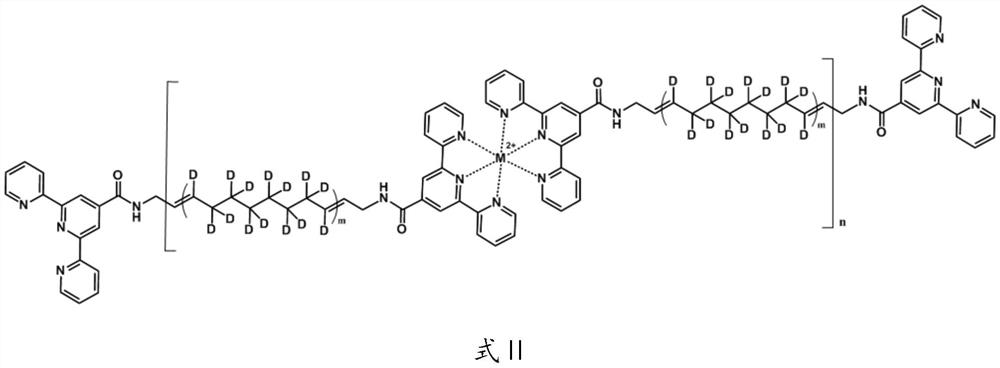
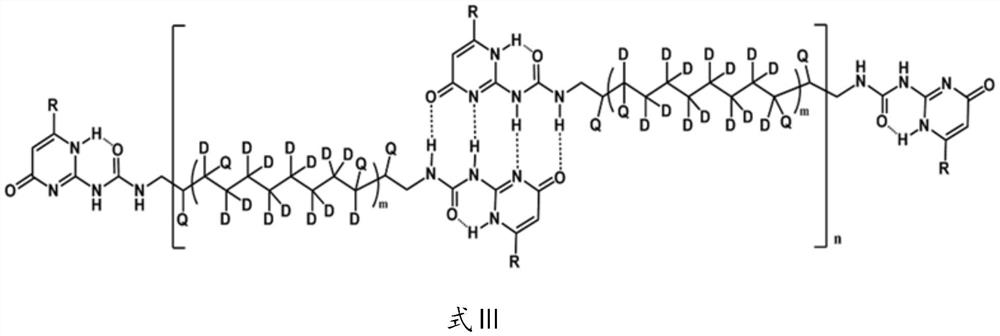
A kind of deuterated supramolecular polymer and preparation method thereof
ActiveCN111440327BEasy to processThe source of raw materials for preparation is simplePolymer scienceCycloalkene
The invention provides a deuterated supramolecular polymer, belonging to the technical field of supramolecular polymers. The deuterated supramolecular polymer is a bifunctional monomer with a deuterated polymer as a spacer obtained after ring-opening metathesis polymerization of a deuterated cycloalkene, through quadruple hydrogen bonds or metal coordination bonds. Quadruple hydrogen bonded deuterated supramolecular polymers or metal coordination deuterated supramolecular polymers formed by non-covalent interactions. The invention also provides a preparation method of the deuterated supramolecular polymer. The main chain of the deuterated supramolecular polymer of the present invention is connected to each other through non-covalent interaction, and has the advantages of self-repair and good processing performance. The preparation source of the deuterated supramolecular polymer of the present invention is simple, the process flow is simple, and the preparation of the deuterated supramolecular polymer with a controllable ratio of unsaturated carbon to saturated carbon can be realized by adjusting the type and molar ratio of deuterated cycloalkene , combined with hydrogen isotope addition reactions can adjust the ratio of hydrogen isotopes in the saturated product.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
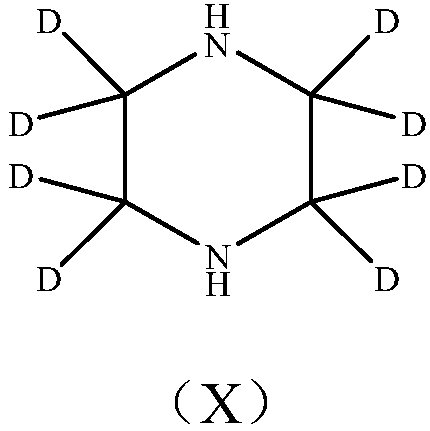
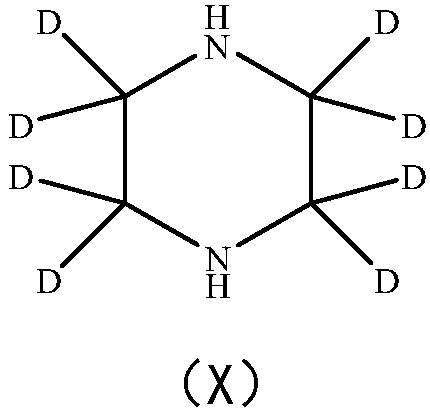
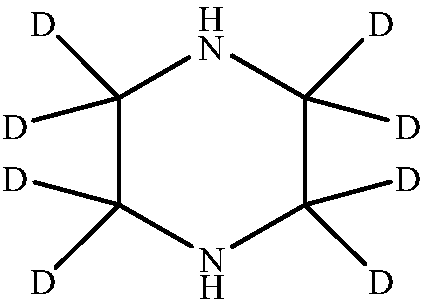
Deuterated piperazine and preparation method thereof
The invention provides deuterated piperazine and a preparation method of the deuterated piperazine, and fills up the technical blank of deuteration of piperazine. The preparation method comprises thefollowing steps: putting piperazine into a liner of a polytetrafluoroethylene reaction kettle, and adding a deuterated reagent; putting the liner into a matched stainless steel kettle body, and tightening a kettle cover by using a bolt; continuously heating at high temperature, controlling the heating temperature to 100-250 DEG C, and controlling the heating time to 24-96 hours; and naturally cooling the polytetrafluoroethylene reaction kettle to the room temperature after finishing heating, transferring a reaction system in the liner to a single-neck flask, conducting vacuum concentration until no solvent exists, and obtaining deuterated piperazine. The molecular structure of the deuterated piperazine is shown in the description.
Owner:天津莱茵泰克生物科技有限公司
A kind of method of synthesizing chiral amine
ActiveCN109111380BHigh yieldHigh stereoselectivityOrganic chemistry methodsMetallocenesArylPtru catalyst
Owner:SHAANXI NORMAL UNIV
Synthesis method of palladium-catalyzed C-1 deuterated aromatic aldehyde
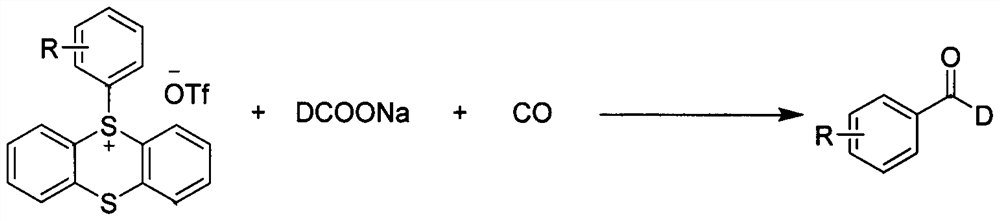
PendingCN114656347AImprove adaptabilityImprove reaction efficiencyOrganic compound preparationOrganic chemistry methodsPtru catalystFormate
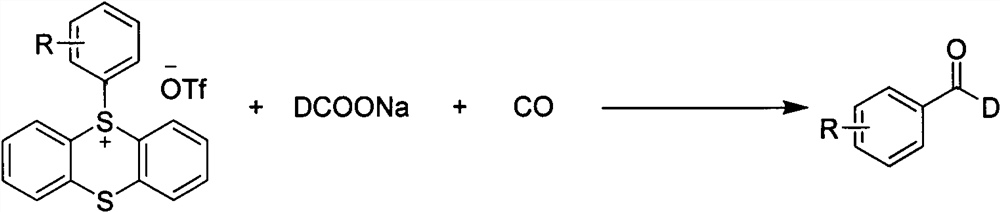
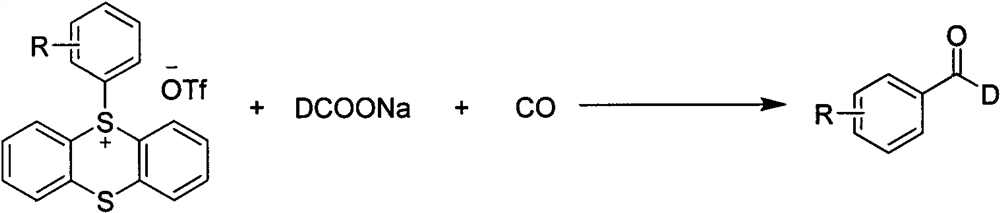
The invention discloses a method for synthesizing C-1 deuterated aromatic aldehydes by catalyzing an aryl sulfosalt compound through palladium, and belongs to the field of synthesis of organic deuterated compounds. The method comprises the following steps: by taking an aryl sulfur salt compound, sodium deuterated formate and carbon monoxide as raw materials, palladium salt as a catalyst and tris (1-naphthyl) phosphine as a ligand, adding triethylamine, and reacting in an N, N-dimethylformamide solvent at 120 DEG C for 12 hours to obtain the C-1 deuterated aromatic aldehyde compound after the reaction is finished. According to the synthesis method, the substrate and the deuteration reagent are cheap and easy to obtain, the reaction efficiency is high, the deuteration rate is high, the adaptability of functional groups is very good, the reaction conditions are simple and convenient to operate, and the method has a good industrial application prospect.
Owner:NANJING FORESTRY UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com