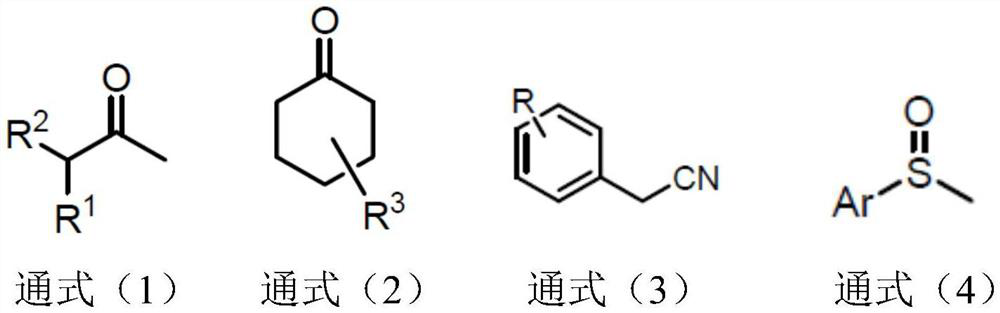
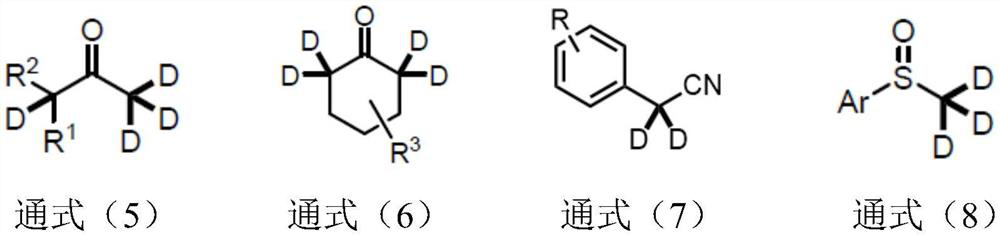
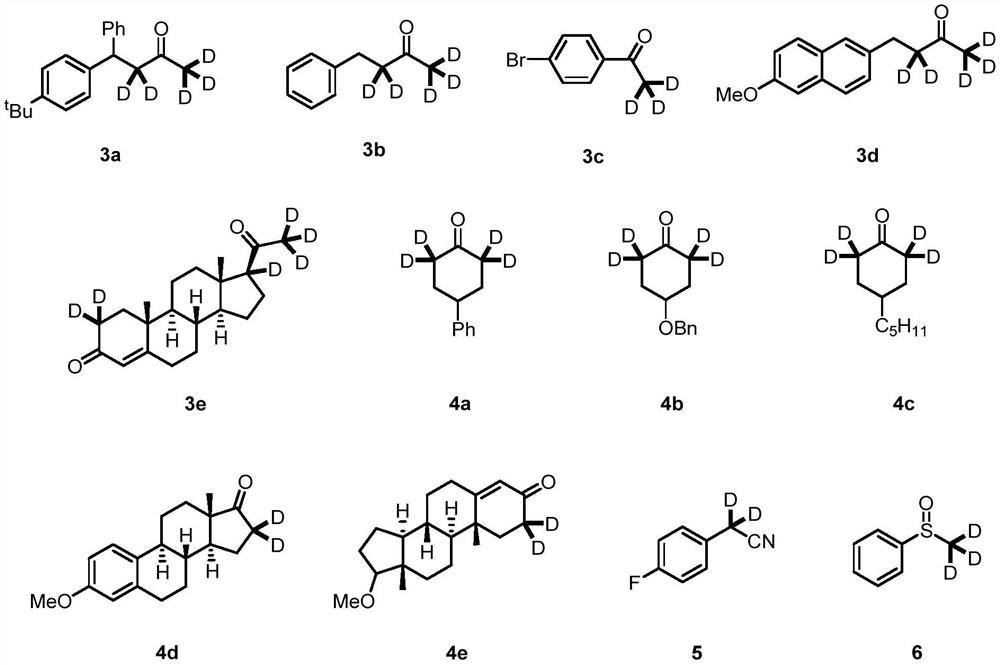
Synthetic method of alpha-deuterated carbonyl compound
A synthesis method and compound technology, applied in the field of deuterated compound preparation, can solve the problems of expensive catalyst, cumbersome post-processing, low yield and the like, and achieve the effects of good regioselectivity, wide application range and high deuteration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Into a 4 mL vial, 15.3 mg (0.1 mmol) of barium oxide, 100 μL (50.0 mmol) of deuterated water, 400 μL of tetrahydrofuran and 56.0 mg (0.1 mmol) of compound 1a were added, and the reaction mixture was stirred at 80° C. for 12 h. Water was then added to quench the reaction. Ethyl acetate and water were added for extraction, and the organic phase was dried and concentrated to obtain the target compound 3a in a yield of 96%. The deuteration rates of the α site (from left to right) were 97% and 95%, respectively.
[0041] The target product 3a obtained by the above-mentioned synthetic method is detected by hydrogen nuclear magnetic resonance spectrum and carbon spectrum, and the test results are as follows: 1 H NMR (300MHz, CDCl 3)δ7.33-7.21(m,6H),7.21-7.09(m,3H),4.53(s,1H),3.14(d,J=5.9Hz,0.08H),2.03(dd,J=5.7,3.4 Hz,0.16H),1.27(s,9H). 13 C NMR (75MHz, CDCl 3 )δ207.4,149.1,144.0,140.7,128.6,127.7,127.2,126.4,125.5,49.3,45.6,34.4,31.3,29.6.
Embodiment 2
[0043]
[0044] Into a 4 mL vial, 15.3 mg (0.1 mmol) of barium oxide, 100 μL (50.0 mmol) of deuterated water, 400 μL of tetrahydrofuran and 29.6 mg (0.1 mmol) of compound 1b were added, and the reaction mixture was stirred at 80° C. for 12 h. Water was then added to quench the reaction. Ethyl acetate and water were added for extraction, and the organic phase was dried and concentrated to obtain the target compound 3b in a yield of 99%. The deuteration rates of the α site (from left to right) were 98% and 93%, respectively. The target product 3b obtained by the above-mentioned synthetic method was detected by hydrogen NMR and carbon spectroscopy, and the test results were as follows: 1 H NMR (300MHz, CDCl 3 )δ7.27(dd,J=9.4,5.1Hz,2H),7.19(t,J=6.4Hz,3H),2.87(s,2H),2.73(dd,J=7.2,2.6Hz,0.07H) ,2.10(dt,J=4.4,2.2Hz,0.18H). 13 C NMR (75MHz, CDCl 3 )δ208.3,141.0,128.5,128.3,126.3,44.7,44.4,44.2,29.6,29.3,29.1,28.9.
Embodiment 3
[0046]
[0047] To a 4 mL vial, 15.3 mg (0.1 mmol) of barium oxide, 100 μL (50.0 mmol) of deuterium oxide, 400 μL of tetrahydrofuran and 39.6 mg (0.1 mmol) of compound 1c were added, and the reaction mixture was stirred at 80° C. for 12 h. Water was then added to quench the reaction. Ethyl acetate and water were added for extraction, and the organic phase was dried and concentrated to obtain the target compound 3c with a yield of 99% and a deuteration rate of α site of 99%.
[0048] The target product 3c obtained by the above-mentioned synthetic method was detected by hydrogen nuclear magnetic resonance spectrum and carbon spectrum, and the test results were as follows: 1 H NMR (300MHz, CDCl 3 )δ7.84-7.82(m,1H),7.81-7.79(m,1H),7.63-7.60(m,1H),7.60-7.57(m,1H),2.55(dt,J=4.5,2.2Hz, 0.03H). 13 C NMR (75MHz, CDCl 3 )δ197.1,135.8,131.9,129.8,128.3,25.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com