A kind of preparation method of nickel-catalyzed α-deuterated chiral sulfonamide compound
A technology for sulfonamides and compounds, which is applied in the field of preparation of α-deuterated chiral sulfonamide compounds, can solve the problems of small reaction scale and range, high price, low selectivity, etc., and save experimental steps and costs, and catalyst consumption Low, high enantioselectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
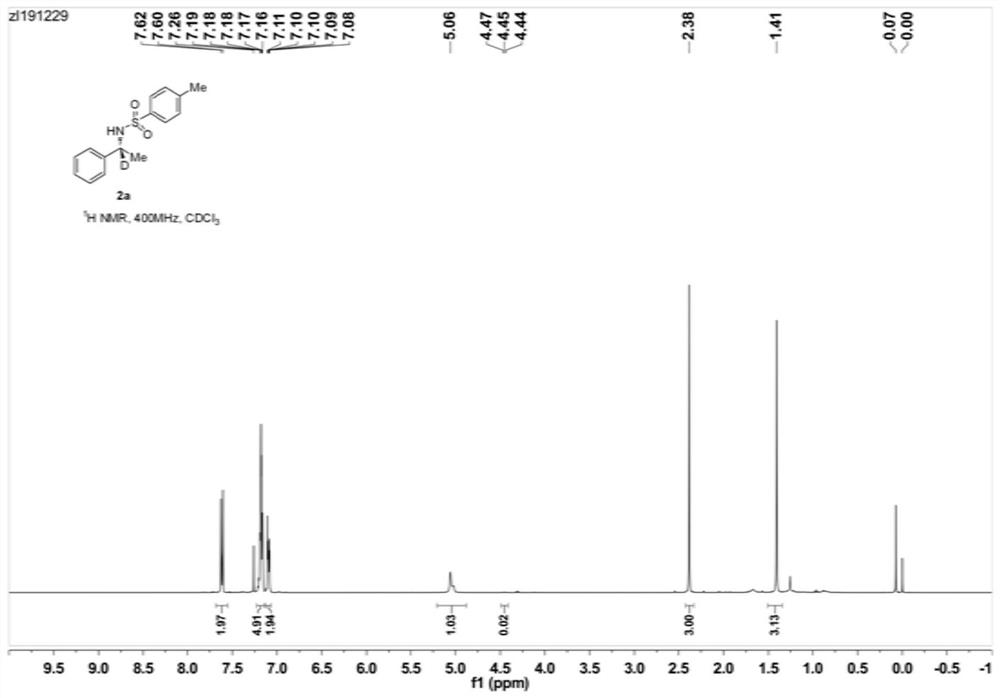
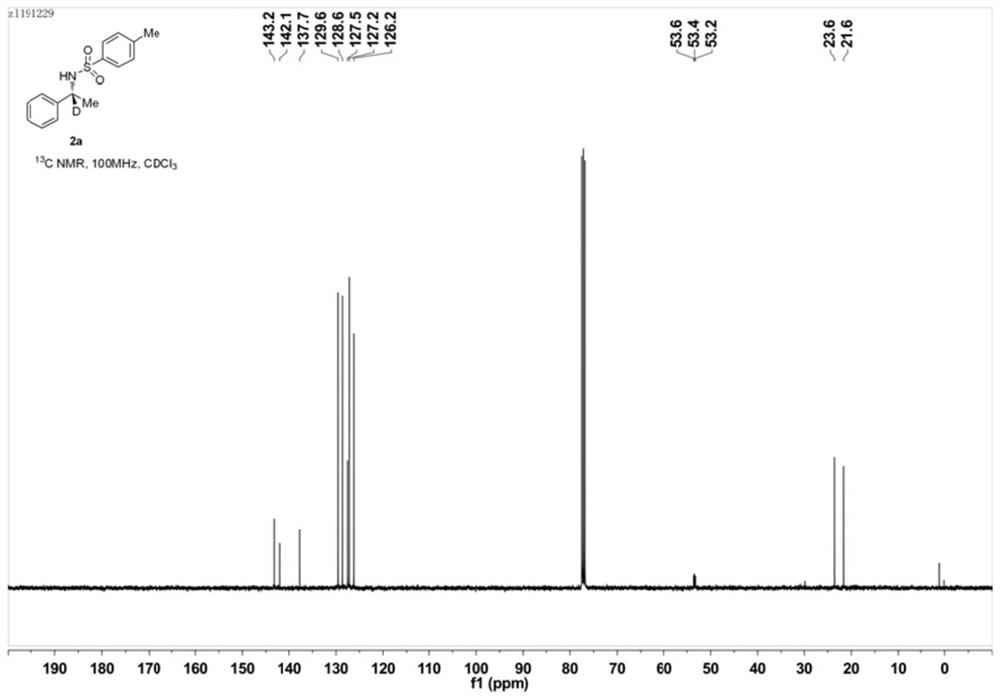
[0052] Example 1: Preparation of (R)-N-tosyl-1-phenyl-1-d-ethylamine (2a)
[0053]
[0054] Under nitrogen protection, add Ni(OTf) into a 10mL Schlenk reaction tube 2 (0.72mg, 0.002mmol), (S)-Binapine (1.84mg, 0.0025mmol), Molecular sieves (50 mg) and isopropanol-d8 (0.3 mL), stirred for 10 minutes, added sulfonylimide 1a (27.4 mg, 0.1 mmol). The reaction tube was sealed and heated in an oil bath at 60°C for 24 hours. After the reaction stopped, cool down, the distillation device recovered the solvent, the residue was added to a silica gel column, and eluted with petroleum ether and ethyl acetate (volume ratio 10:1) to obtain pure (R)-N-p-toluenesulfonyl-1- Phenyl-1-d-ethylamine (2a), yield 99%, enantiomeric excess (ee) 94%, deuterated rate>98%. NMR, HPLC, and high-resolution mass spectrometry data are as follows:
[0055] 1 H NMR (400MHz, CDCl 3 ): δ7.61(d, J=8.3Hz, 2H), 7.19-7.16(m, 5H), 7.11-7.08(m, 2H), 5.06(s, 1H, NH), 4.45(pseudopentet, J=7.1 Hz, 0.02H), 2.38(...
Embodiment 2
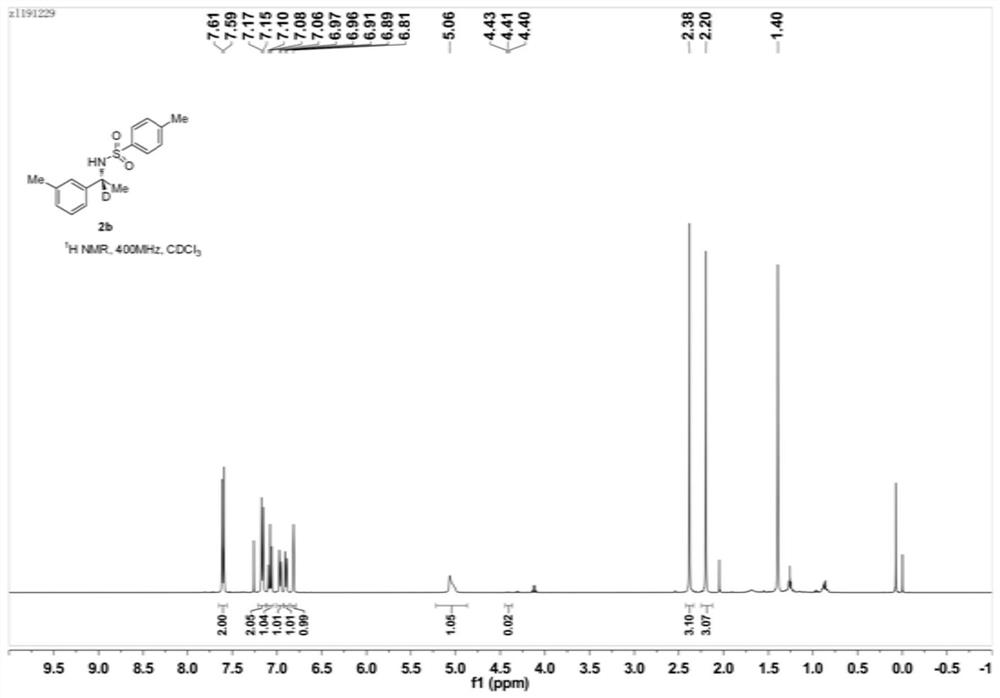
[0059] Example 2: Preparation of (R)-N-p-toluenesulfonyl-1-(3-methylphenyl)-1-d-ethylamine (2b)
[0060]
[0061] Under nitrogen protection, add Ni(OTf) into a 10mL Schlenk reaction tube 2 (0.72mg, 0.002mmol), (S)-Binapine (1.84mg, 0.0025mmol), Molecular sieves (5 mg) and isopropanol-d8 (0.3 mL), stirred for 10 minutes, added sulfonylimide 1b (28.8 mg, 0.1 mmol). The reaction tube was sealed and heated in an oil bath at 60°C for 24 hours. After the reaction stopped, cool down, the distillation device recovered the solvent, the residue was added to a silica gel column, and eluted with petroleum ether and ethyl acetate (volume ratio 10:1) to obtain pure (R)-N-p-toluenesulfonyl-1- (3-methylphenyl)-1-d-ethylamine (2b), yield 99%, enantiomeric excess (ee) 93%, deuteration rate 98%. NMR, HPLC, and high-resolution mass spectrometry data are as follows:
[0062] 1 H NMR (400MHz, CDCl 3 ): δ7.60(d, J=8.3Hz, 2H), 7.16(d, J=8.1Hz, 2H), 7.08(t, J=7.6Hz, 1H), 6.96(d, J=7.5Hz, 1H ...
Embodiment 3
[0066] Example 3: Preparation of (R)-N-p-toluenesulfonyl-1-(4-fluorophenyl)-1-d-ethylamine (2c)
[0067]
[0068] Under nitrogen protection, add Ni(OTf) into a 10mL Schlenk reaction tube 2 (0.72mg, 0.002mmol), (S)-Binapine (1.84mg, 0.0025mmol), Molecular sieves (5 mg) and isopropanol-d8 (0.3 mL), stirred for 10 minutes, and sulfonylimide 1c (29.2 mg, 0.1 mmol) was added. The reaction tube was sealed and heated in an oil bath at 60°C for 24 hours. After the reaction stopped, cool down, the distillation device recovered the solvent, the residue was added to a silica gel column, and eluted with petroleum ether and ethyl acetate (volume ratio 10:1) to obtain pure (R)-N-p-toluenesulfonyl-1- (4-fluorophenyl)-1-d-ethylamine (2c), yield 99%, enantiomeric excess (ee) 93%, deuteration rate 97%. NMR, HPLC, and high-resolution mass spectrometry data are as follows:
[0069] 1 H NMR (400MHz, CDCl 3): δ7.59(d, J=8.2Hz, 2H), 7.18(d, J=8.0Hz, 2H), 7.11-7.01(m, 2H), 6.85(m, 2H), 5.22...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com