N-deuterium methylamine compound and preparation method thereof
A technology of amine compounds and deuteromethylamine, applied in the field of N-deuteromethylamine compounds and their preparation, can solve the problems of low selectivity, high cost, high toxicity of deuteromethylation reagents, etc. High efficiency, reduce pollution and waste, avoid the effect of highly toxic reagents and highly dangerous reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
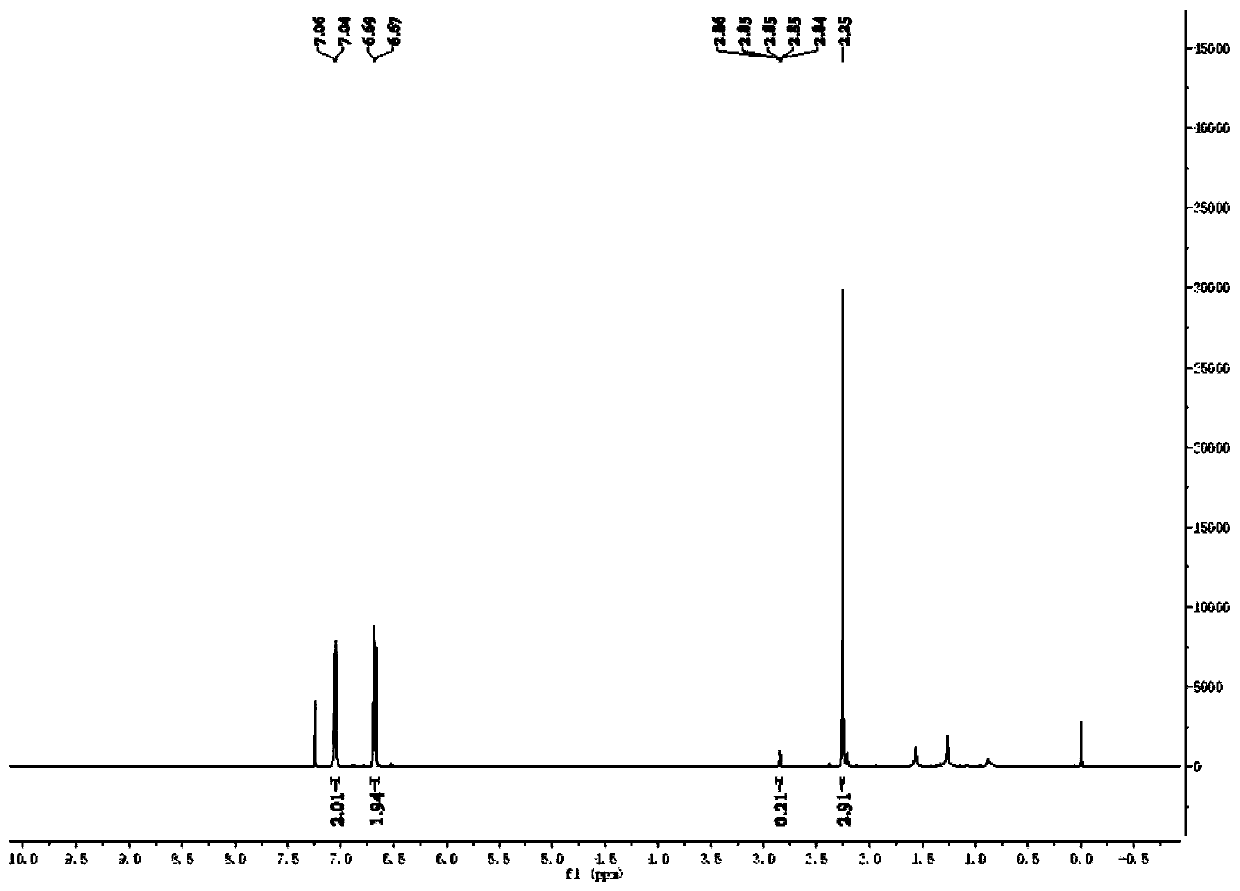
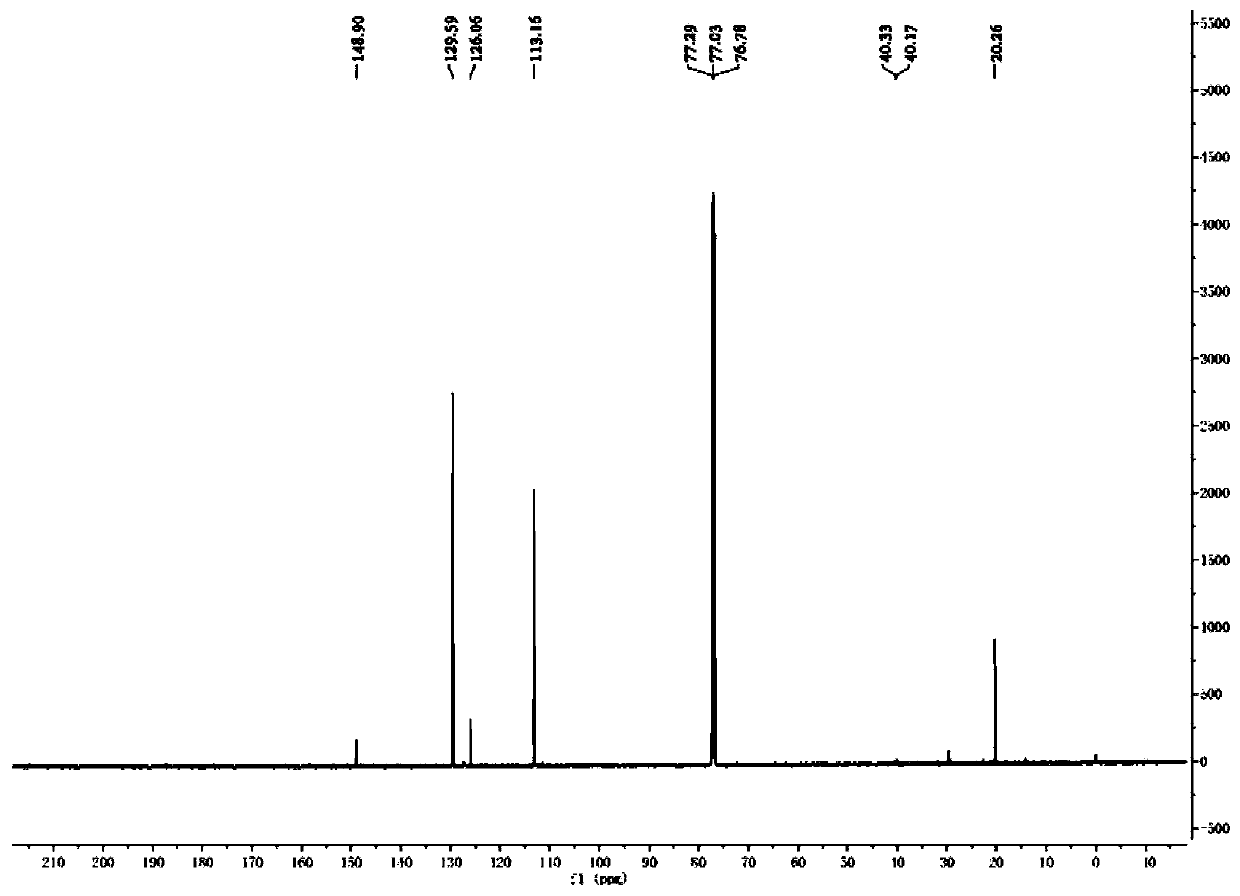
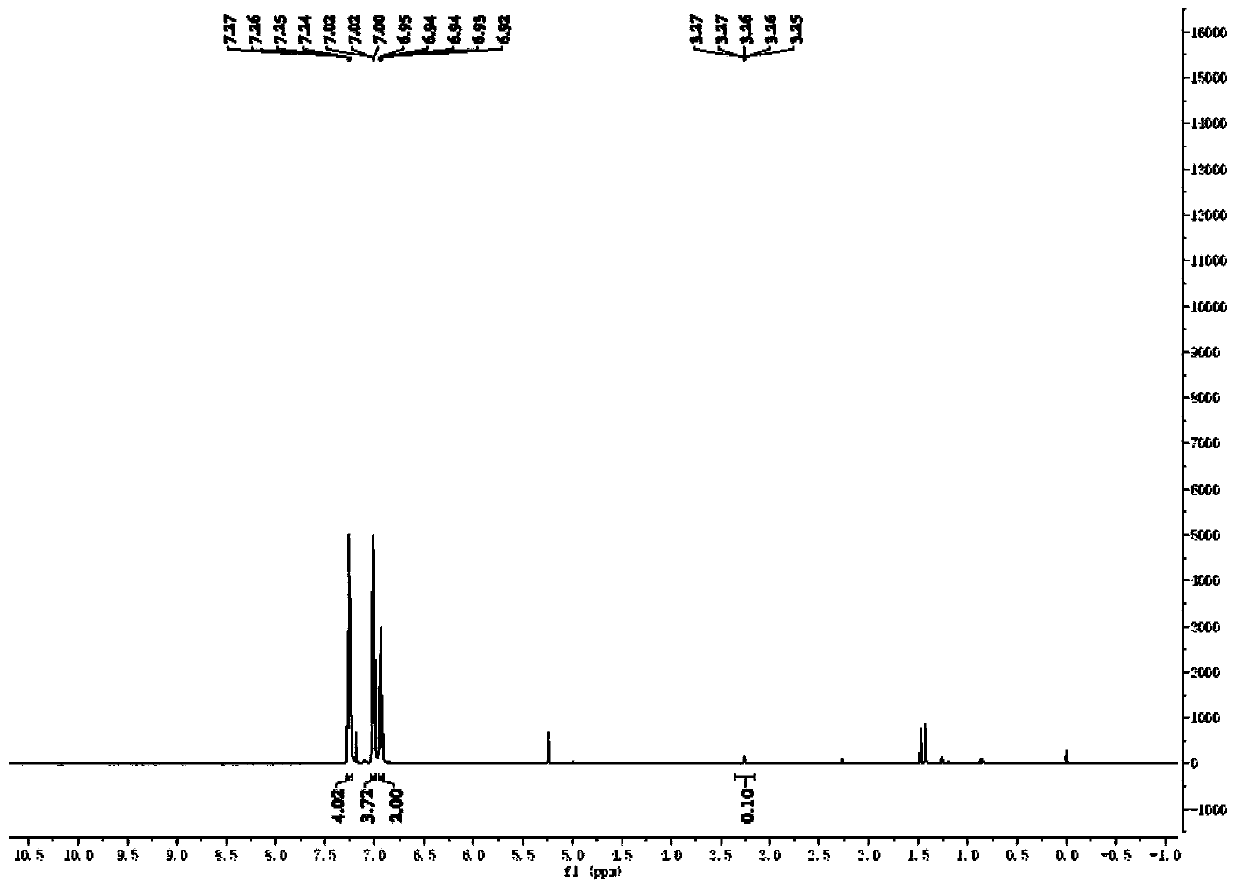
[0050] Weigh 0.3mmol of p-methylaniline and 10.0mg of Pd / PCN photocatalyst into a 5mL reaction flask, and add a mixed solution of deuterium water / deuterated methanol (1.5mL / 1.5mL) to replace the reaction system with argon Protected state, then place the reaction bottle under a 420nm light source for light reaction for 4 hours, remove the light source after the reaction, and dilute the reaction mixture with 5.0mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. The solvent is removed by rotary evaporation, and then the pure target product is obtained by column chromatography (developing solvent: ethyl acetate / petroleum ether), such as Figure 1-2 shown by 1 HNMR, C-NMR and other tests confirm the structure, the yield is 89%, and the deuteration rate is >97%.
Embodiment 2
[0052]
[0053] Weigh 0.3mmol of p-methylaniline and 10.0mg of Pd / PCN photocatalyst into a 5mL reaction bottle, and add a mixed solution of water / deuterated methanol (1.5mL / 1.5mL) to replace the reaction system with argon protection state, then place the reaction bottle under a 420nm light source for 4 hours of light reaction, remove the light source after the reaction, and dilute the reaction mixture with 5.0mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. The solvent is removed by rotary evaporation, and then the pure target product is obtained by column chromatography (developing solvent: ethyl acetate / petroleum ether). 1 HNMR, C-NMR and other tests confirm the structure, the yield is 91%, and the deuteration rate is >99%.
Embodiment 3
[0055]
[0056] Weigh 0.3mmol of p-methylaniline and 10.0mg of Pd / PCN photocatalyst into a 5mL reaction bottle, and add a mixed solution of deuterium water / methanol (1.5mL / 1.5mL) to replace the reaction system with argon protection state , and then the reaction bottle was placed under a 420nm light source for 4 hours of light reaction. After the reaction, the light source was removed, and the reaction mixture was washed with 5.0mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. The solvent is removed by rotary evaporation, and then the pure target product is obtained by column chromatography (developing solvent: ethyl acetate / petroleum ether). 1 HNMR, C-NMR and other tests confirm the structure, the yield is 91%, and the deuteration rate is >99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com